AN INVESTIGATION OF THE REMOVABILITY OF NATURALLY AGED SYNTHETIC PICTURE VARNISHESSUZANNE QUILLEN LOMAX, & SARAH L. FISHER
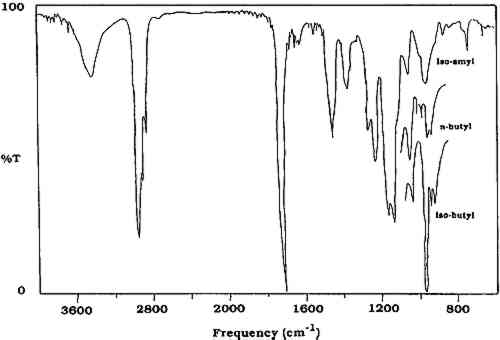
ABSTRACT—The removability of naturally aged synthetic picture varnishes has been examined on more than 50 paintings. Most of these paintings had been coated with either poly(isoamyl methacrylate) or poly(n-butyl methacrylate). Since these aged methacrylate coatings were applied between 10 and 40 years ago, questions had been posed about possible cross-linking of the polymer chains, which would make them more difficult to remove with solvents or even render them completely insoluble. These questions were investigated by applying solvents of increasing polarity to the varnish by swab, and analyzing the material removed by infrared spectroscopy. All coatings were found to be removable with solvents, although often fairly polar solvent mixtures were necessary.Only 5 of 25 paintings suspected of being coated with poly(isoamyl methacrylate) were found to be coated with this varnish. The varnish, a type known as 27H, was removed with relatively mild solvents, often by swelling. Thirty-one paintings coated with poly(n-butyl methacrylate) were examined. Although solvents more polar than acetone were not required for removal of the coatings, concerns were raised about the sensitivity of the paint films of early American paintings to that solvent. In addition, although these coatings are removable today, the future aging behavior of these varnishes under the conditions of exposure at the National Gallery of Art, where ultraviolet filters are in place, has not been determined. Continued systematic monitoring of the aging behavior of these varnishes on selected paintings will be carried out. 1 INTRODUCTIONTHE REMOVABILITY of two aging methacrylate varnishes, poly(isoamyl methacrylate), known as 27H, and various poly(n-butyl methacrylate)s, has been a concern at the National Gallery of Art due to the relatively large number of paintings covered with these coatings and the knowledge that they cross-link in accelerated aging experiments, rendering them insoluble (Feller et al. 1981). The traditional natural resin varnishes, such as dammar resin and gum mastic, form brittle, yellowed films as they deteriorate (Feller 1958; Constable 1979; Thomson 1957; LaFontaine 1979). It has been noted that photochemical autoxidation is the method by which these natural resins undergo degradation (de la Rie 1987b, 1988). In general, the oxidation proceeds to a degree at which time toluene-acetone mixtures (40:60 v:v) are required for varnish removal (Feller and Curran 1975). On the other hand, some synthetic varnishes are suspect under the principle of reversibility (AIC 1985), requires that materials used in a conservation treatment, such as a picture varnish, should be removable without damage to the object (Gettens 1935; Feller 1960, 1959, 1957b). Some also seem to violate the principle of having the appropriate aesthetic qualities (de la Rie 1987a; De Witte et al. 1981). All natural and synthetic varnishes tend to be less soluble in solvents of low polarity over time due to oxidation. However, synthetic varnishes may also become less soluble due to the phenomenon of cross-linking. Most synthetic varnishes are made up of long-chain polymeric structures consisting of molecules of hundreds of repeating units. If these long chains become chemically linked, the polymer eventually becomes insoluble in the solvents in which it was originally soluble. When the number of cross-links is relatively low, the polymer can be removed by swelling it by the proper solvent, followed by mechanical removal. If the degree of cross-linking has progressed to an advanced state, this method of removal is no longer possible. The cross-linking reaction is activated by ultraviolet radiation (Morimoto and Suzuki 1972), but it has also been shown to proceed in the presence of visible light (Feller et al. 1981; Feller 1967). It has been observed, on the basis of artificial aging studies, that the methacrylate polymers most sensitive to cross-linking have the alkyl side chains of isoamyl, isobutyl, 2-methylbutyl, and n-butyl (Feller et al. 1981; Feller et al. 1985). Side chains possessing tertiary hydrogen atoms exhibit greater reactivity in the cross-linking process, as these hydrogen atoms are more readily abstracted by the free radical species involved in the process. The two varnishes examined in this study are 27H (poly(isoamyl methacrylate)) and poly(n-butyl methacrylate). According to the conservation records, 27H was reported to have been applied to 92 paintings in the period 1952–57 at the National Gallery of Art. Most of these are “old master” paintings that at least since 1952 have been exposed to light under normal exhibition conditions. In this paper, the term “old master” refers to works by great European artists of the 16th to 18th centuries. The conservation records also show that a poly(n-butyl methacrylate) varnish was applied to 184 additional paintings now in the National Gallery of Art collection. This varnish was applied between 10 and 40 years ago either by National Gallery of Art conservators or by private conservators in the case of paintings not then in the gallery collection. The varnish 27H was developed in the early 1950s as part of a Mellon Institute research project at the National Gallery of Art (Feller and Raynolds 1953). The resin was never commercially manufactured but was prepared in batches and distributed to selected conservators. It was observed in 1955 that 27H did not completely depolymerize on heating, a situation attributed to cross-linking of the polymer (Feller 1955a). (An excellent review of the cross-linking studies of 27H and other methacrylate polymers can be found in Feller et al. 1985, appendix A.) Samples were exposed to accelerated aging conditions (National Accelerated Fading Unit, Type XV, Corex filter) and were subsequently found to be insoluble in mild solvents such as hexane, benzene, or toluene (Feller 1955b). UV absorbers were added (Feller 1956), among them Uvinul 400 and Uvinul D-49, but their use was discontinued because they led to yellowing of the varnish, and the original formulation of 27H was no longer produced after 1957 (Feller 1957b). Poly(n-butyl methacrylate) has been used as a picture varnish since the 1930s. It is known under a wide variety of trade names, including Lucite 44 (DuPont), Elvacite 2044 (DuPont), Acryloid F-10 (Rohm and Haas), Acryloid B-66 (Rohm and Haas, a terpolymer of poly(n-butyl methacrylate) and an ethyl methacrylate/methyl acrylate copolymer) (Feller 1971–72), M varnish (Ralph Mayer), Soluvar (Permanent Pigments) (Feller 1973–74), and Synvar (Weber). Feller et al. (1981) has suggested that there is an induction time of 11 years before insoluble matter begins to form in commercial n-butyl and isobutyl polymers on well-illuminated museum walls. Testing revealed that n-butyl and isobutyl methacrylate polymers develop 50–80% insoluble matter after exposure to 9 million footcandle hours of radiation from daylight-type fluorescent lamps. This exposure is approximately equal to 50 years of exposure to daylight under an average illumination level of 50 footcandles. Although ultraviolet filters (Plexiglas UF3) were installed at the National In an attempt to understand the aging behavior of these coatings, removability studies were carried out on paintings believed to be varnished with them. It should be pointed out that these tests were undertaken only to determine qualitatively the removability of the varnish. Neither the degree of cross-linking nor the amount of insoluble material was determined, but rather the solvent required to remove the varnish. It is obvious that some insoluble material can be removed by the mechanical action of rubbing (Feller and Curran 1975). 2 INFRARED SPECTROSCOPYIN THIS study, the analytical tool selected for identifying varnish removed from paintings was infrared spectroscopy. Pyrolysis gas chromatography has been used extensively to qualitatively and quantitatively examine acrylic polymers (Radell and Strutz 1959; Lehmann and Brauer 1961; Lehrle and Robb 1967; Esposito and Swann 1965; Ettre and Varadi 1962). Frankoski and Siggia (1972) have also examined alkali fusion reaction gas chromatography in the study of polyacrylates and polymethacrylates. Based on instrumentation available in our laboratory, however, and on ease of sample manipulation and analysis, infrared spectroscopy was employed to identify the synthetic varnishes removed from paintings. This technique allows for the analysis of organic molecules by identification of basic structural components. A Mattson Alpha Centauri Fourier Transform Infrared (FTIR) spectrometer was used for all analyses. The varnish material removed by swab was extracted with methylene chloride, and the resulting solution was concentrated and spotted on a KBr pellet for infrared analysis. This FTIR technique allows the detection of traces of resins on the swab that could not be visually detected. A literature search of the Mellon Institute Quarterly Reports(Feller 1956) indicated that standard aged varnish samples had been placed above the laylights of the National Gallery of Art in 1956. This is an uninsulated area between the interior skylights of the galleries and the roof of the building. Although no specific aging conditions were reported for these samples, they were exposed to both unfiltered daylight and extremes of temperature. It was roughly estimated (Feller 1959) that exposure in the “attic,” above the laylights, is about 15 times that in the galleries. Eleven of these aged samples were available. In order to supplement the collection of naturally aged materials, contact was made with Marjorie B. Cohn, former head conservator at the Center for Conservation and Technical Studies, Fogg Art Museum. Permission was given for the collection there to be sampled, and more than 150 naturally aged samples dating from 1933 to 1946, including natural and synthetic resins, oil-varnish mixtures, and other media, were collected. In order to have additional reference materials, samples of natural and synthetic coatings for artificial (thermal) and natural aging were prepared at the National Gallery of Art. Glass panels or slides were coated with films (thickness not measured) of various varnishes. Each sample was prepared in duplicate. One series was placed in a dry circulating oven at 100�C for more than one year, and the other was placed against a wall in the laboratory exposed to fluorescent lights for nine hours a day for more than one year. Samples from the oven-aged panels were used to compare changes in fluorescence, solubility, and infrared spectra upon aging. The samples designated for natural aging were used as controls. All of these unaged, naturally aged, and artificially aged varnishes of known composition were examined by infrared spectroscopy to obtain a data base against which unknowns could be compared. Aged samples of the natural resins dammar and mastic are too similar to be distinguished by infrared spectroscopy. Infrared spectra of the naturally aged (Fogg) and oven-aged samples of these two natural resins show virtually the same absorbances and intensities. Both of these materials have carbonyl (C=O) absorbances in the region of 1715–1700 cm−1, as well as multiple absorbances in the region 1270–1000 cm−1. Polycyclohexanone resins such as Laropal K-80 and AW-2 (BASF) also have carbonyl absorption in the region 1715–1700 cm−1, as does Rembrandt varnish (Talens). However, these materials are characterized by lack of an absorbance in the region of 1380 cm−1, which is present in dammar and mastic, as well as by the presence of a strong absorption at 1060 cm−1. Hydrogenated polycyclohexanone such as MS-2A possesses a strong absorbance at 3420 cm−1. The methacrylate polymers and other synthetic polymers have carbonyl absorbances in the region of 1740–1730 cm−1(Sadtler Research Laboratories 1980). The infrared spectra of these methacrylate polymers do not change substantially upon aging. A slight loss of resolution is often observed, but the absorbances and intensities remain constant. The methacrylate polymers can be readily distinguished from poly(vinyl acetate) because the latter possesses one strong absorbance at 1240 cm−1(table 1). The methacrylate polymers have a weaker double band at 1269 and 1241 cm−1. In addition, the region from 1175 to 970 cm−1 is different for the two classes of polymers. Poly(vinyl alcohol) is easily identified by the strong O-H absorbance at 3350 cm−1(Thomson 1963). TABLE 1 Selected Infrared Absorbances of Natural and Synthetic Varnishes (in cm−1) Distinguishing between the methacrylate polymers can often be difficult, especially if the sample is small or is “contaminated” with other varnishes. Fortunately, n-butyl, isobutyl, and isoamyl methacrylate homopolymers can be distinguished by comparing the regions from 1000–950 cm−1. As shown in figure 1, the spectrum of the n-butyl polymer consists of two doublets, while the isobutyl polymer has a triplet, with one of the three peaks being much more intense than the other two. The spectrum of the isoamyl polymer is characterized by a stronger single absorbance at 970 cm−1 with a small peak at 1130 cm−1. Therefore, it usually is possible to distinguish between these three methacrylate polymers by infrared spectroscopy, provided that the spectrum is sufficiently resolved in the region of 1000 to 950 cm−1. Usually a small amount of poly(isobutyl methacrylate) is found as an impurity in poly(n-butyl methacrylate), and its presence can be detected in most cases in the infrared spectrum.
3 DESCRIPTION OF TESTING PROCEDUREPAINTINGS WERE selected on the basis of the simplicity of varnish stratification and the age of the coating, as indicated by the National Gallery of Art's conservation files. A representative sampling of at least 25 paintings believed to be coated with each varnish was selected. The exhibition history of each painting was examined to compare the time exposed to light under exhibition conditions to the time in storage, since cross-linking is a light-induced phenomenon. The paintings were examined under ultraviolet light, where areas of retouch were noted (de la Rie 1982), as was the color of the fluorescence. Most natural resins fluoresce green under long-wavelength ultraviolet light (a notable exception is shellac, which fluoresces orange), while synthetic resins often have a milky fluorescence. The paintings' appearance under visible light was also carefully noted in an effort to keep a record, albeit subjective, of the aesthetic appearance of these 30-year-old synthetics. At least two test spots were selected on each painting, usually one in a light area and one in a dark area. Solvents of increasing polarity were applied to the areas with swabs, while the areas were viewed under a stereo microscope. In order of increasing polarity, the solvents used were: cyclohexane, cyclohexane-toluene mixtures, pure toluene, toluene-acetone mixtures, pure acetone (Feller 1978, 1976; Feller et al. 1985), and a mixture of isopropanol and methyl ethyl ketone (MEK) (table 2). The mildest solvent was applied first and the solvent progression was continued until the varnishes were completely removed, as monitored by infrared spectroscopy. TABLE 2 Solvent mixtures for removability studies, with dispersion factors Following the application of solvents, changes in the varnish were noted, including blanching, matteness, and gelling. The observations were recorded in a standard format, and reports were compiled on each painting. The removal of all of the varnish layers was determined by visual examination and by comparison of the fluorescence of the spot with the surrounding area. The swabs were saved and independently analyzed by FTIR. 4 RESULTS—POLY(ISOAMYL METHACRYLATE), 27HTWENTY-FIVE PAINTINGS believed on the basis of National Gallery of Art records to be coated with 27H were tested. Only 5 paintings were found to actually be coated with 27H. This unexpected result is possibly attributable to incomplete documentation of subsequent treatments of the other 20 paintings before the establishment of a formal conservation facility at the National Gallery. An additional 5 of the 25 paintings were coated with varnishes that were identified as methacrylate polymers, perhaps 27H. In these cases, the resolution of the infrared spectra obtained on samples of the varnishes was not sufficient to permit a determination of which methacrylate polymer was present. One of these 5 paintings, which was of expendable quality, had been exposed to unfiltered sunlight and temperature extremes above the gallery laylights as a test for 14 to 24 years. As previously stated, exposure in the “attic” was estimated to be about 15 times that in the gallery, making comparisons with other samples difficult but possibly of interest. The coating was in such an advanced state of degradation that no solvent could remove it. Scrapings of a small quantity of the varnish were used in the infrared analysis to identify the material as a methacrylate polymer. The infrared spectrum of the coating on another of these five paintings revealed that it is a mixture of a methacrylate polymer and a natural resin. Twelve of these 25 paintings were found to have poly(vinyl acetate) as a coating; in many of these the coating had been applied over a natural resin. One of the coatings was On the five paintings that were found to actually be coated with 27H, gel formation occurred with application of a mixture of 60% cyclohexane, 40% toluene. In four cases, the gel was soft and rubbery, although in one case it was particulate. Application of 100% toluene caused the formation of additional gel in all of the cases, with the gel forming more rapidly in the more polar solvent. In two of the cases, toluene removed all of the 27H. In the other three, mixtures of toluene and acetone removed the 27H as a gel on two paintings, whereas pure acetone was required for complete varnish removal on one painting, as determined by infrared spectroscopy on cleaning swab residues. In all of the cases, the 27H was found over a coat of either a natural resin or a Rembrandt varnish. These varnishes were usually removed in acetone-toluene mixtures or pure acetone. 5 RESULTS—POLY(N-BUTYL METHACRYLATE)TWO DIFFERENT types of paintings were examined in this study. One group consists of “old master” paintings to which coatings of Elvacite 2044 were applied in the 1970s and which have been on continuous exhibit under Plexiglas UF3 filtered daylight. The other group contains early American naive paintings to which the coatings were applied in the 1950s. Although these American paintings have been primarily in storage, many have been exposed to light in occasional loan exhibitions. The unpredictable solvent sensitivity of the paint layers on these paintings made them of special concern with regard to varnish removability. Thirty-one paintings with poly(n-butyl methacrylate) coatings were examined: 9 “old masters” and 22 early American paintings. On the “old master” paintings, a soft and rubbery gel formation occurred immediately either with cyclohexane or a mixture of cyclohexane and toluene. More varnish was removed with toluene. On three of the paintings, toluene alone was sufficient to remove all of the poly(n-butyl methacrylate). On two paintings, the poly(n-butyl methacrylate) had been applied over a coat of B-67, which is poly(isobutyl methacrylate); toluene-acetone mixtures (60:40) removed this lower layer. On the remaining paintings, removal of poly(n-butyl methacrylate) continued and was only completed when acetone was applied. On almost all of the 22 early American paintings, a mixture of 60% cyclohexane, 40% toluene, applied by swab, began to remove the varnish. Usually a soft, rubbery gel formed. More varnish was removed with toluene, and, in most cases, the varnish was completely removed in 60% toluene, 40% acetone. The poly(n-butyl methacrylate) was always found over a layer of another varnish, usually a natural resin, and this lower layer could usually be removed with acetone. However, on two of these early American paintings, pigments began to be removed with the acetone-toluene mixture or with pure acetone. These pigments were all in dark areas. Before considering this group of paintings as representative of poly(n-butyl methacrylate) behavior in time, an important fact must be considered: The paintings consist either of cases where the coatings were recently applied and the paintings have been on exhibition, or the coatings were applied 35 to 40 years ago and the paintings have been kept in storage. The individual paintings were chosen for the simplicity of the coating stratification. Since the amount of light is an important factor in the degradation of coatings, 6 CONCLUSIONSIT HAS been shown previously on the basis of accelerated aging studies that certain methacrylate polymers will tend to cross-link and lose their ability to be removed efficiently. In order to monitor this effect, a program of varnish removability testing was initiated. A combination of infrared spectroscopy, visual examination, and fluorescence in ultraviolet light was used to monitor removability of poly(isoamyl methacrylate) and poly(n-butyl methacrylate) varnish coatings. The results of these tests show that some cross-linking of these varnishes has occurred under natural aging conditions with ultraviolet-free light, but that although the varnishes were applied between 10 and 40 years ago, they are still removable. Usually the application of solvents such as cyclohexane-toluene mixtures causes the varnish to gel but does not remove the varnish completely. Solvents of higher polarity (toluene or toluene-acetone mixtures) were necessary for complete, effective varnish removal. The word effective needs to be stressed here. Although a varnish may be eventually removed in a milder solvent, a slightly stronger solvent may be preferred to avoid the need for excessively vigorous rubbing with the swab. It must also be noted that this qualitative removability test does not indicate the amount of cross-linked material or the expected lifetime of the coating. The insolubility of the varnish on the painting exposed to unfiltered sunlight above the laylights demonstrates the effect of ultraviolet filters and reduced light levels. The National Gallery of Art has ultraviolet filters over the skylights. The fact that these varnishes could be removed after 30 to 40 years does not imply that varnishes on paintings in other galleries that do not have these filters would be as easily removed after this exposure time. Care should be taken, therefore, in extrapolating this observation to paintings in other collections. Nor should the observation be interpreted as proof that varnishes remain removable indefinitely. Based on the published results for accelerated aging tests (Feller et al. 1985), it must be assumed that varnishes will eventually become insoluble. Continued monitoring of selected paintings at regular intervals will therefore be undertaken at the National Gallery of Art. ACKNOWLEDGEMENTSJIA-SUN TSANG AND JOHANNE PERRON were members of the initial study group formed to investigate the synthetic surface coatings. Although they have both since left the National Gallery of Art, their hard work, enthusiasm, and dedication are gratefully acknowledged. The substance of this paper was presented to the Paintings Group at the 1988 meeting of the American Institute for Conservation. REFERENCESAIC. 1985. Code of Ethics and Standards of Practice. Washington, D.C.: American Institute for Conservation of Historic and Artistic Works. Constable, W. G.1979. The painter's workshop. 1953. New York: Dover Books.
de laRie, E. R.1982. Fluorescence of paint and varnish layers, pts. 1, 2, and 3. Studies in Conservation27:1–7, 65–69, 102–8. de laRie, E. R.1987a. The influence of varnishes on the appearance of paintings. Studies in Conservation32:1–13. de laRie, E. R.1987b. Research on picture varnishes: Status of the project at the Metropolitan Museum of Art. ICOM Committee for Conservation preprints, 8th Triennial Meeting, Sydney. 2:791–96. de laRie, E. R.1988. Photochemical and thermal degradation of films of dammar resin. Studies in Conservation33:53–71. DeWitte, E., M.Goessens-Landrie, E. J.Goethals, K.VanLerberghe, and C.VanSpringel. 1981. Synthesis of an acrylic varnish with high refractive index. ICOM Committee for Conservation preprints, 6th Triennial Meeting, Ottawa. 81/16/4-1–81/16/4-7. Esposito, G. G., and M. H.Swann. 1965. Application of pyrolysis and programmed temperature gas chromatography to the analysis of thermosetting acrylic coating resins. Journal of Gas Chromatography3:282. Ettre, K., and P. F.Varadi. 1962. Pyrolysis-gas chromatographic technique for direct analysis of thermal degradation products of polymers. Analytical Chemistry34:752. Feller, R. L.1955a. Quarterly report of the Mellon Institute, April–August:18, 20. Feller, R. L.1955b. Quarterly report of the Mellon Institute, September–November:3. Feller, R. L.1956. Quarterly report of the Mellon Institute, June–August:2, 9. Feller, R. L.1957a. Cross-linking of methacrylate polymers by ultraviolet radiation. Paper presented at the New York meeting of the Division of Paint, Plastics and Printing Ink Chemistry, American Chemical Society. 465–70. Feller, R. L.1957b. Quarterly report of the Mellon Institute, June–August:5. Feller, R. L.1958. Dammar and mastic varnishes: Hardness, brittleness, and change in weight upon drying. Studies in Conservation3:162–73. Feller, R. L.1959. Quarterly report of the Mellon Institute, September–November:19. Feller, R. L.1960. Quarterly report of the Mellon Institute, October–December:1. Feller, R. L.1961. Quarterly report of the Mellon Institute, September–November:5–6. Feller, R. L.1967. Research on durable thermoplastic polymers for the conservation of works of art. Estratto Dagli Atti Della XLIX Riunione Della SIPS, Siena. 1099–1102. Feller, R. L.1971–72. Quarterly report of the Mellon Institute, November 1971–April 1972:5–6.
Feller, R. L.1973–74. Quarterly report of the Mellon Institute, November 1973–April 1974:12–13. Feller, R. L.1976. The relative solvent power needed to remove various aged solvent-type coatings. In Conservation and restoration of pictorial art, ed.N.Brommelle and P.Smith. London: Butterworths. 158–161. Feller, R. L.1978. Standards in the evaluation of thermoplastic resins. ICOM Committee for Conservation Preprints, 5th Triennial Meeting, Zagreb. 78/16/4. Feller, R. L., and M.Curran. 1975. Changes in solubility and removability of varnish resins with age. Bulletin of the American Institute for Conservation of Historic and Artistic Works15(2):17–26. Feller, R. L., M.Curran, and C.Bailie. 1981. Photochemical studies of methacrylate coatings for the conservation of museum objects. In Photodegradation and Photostabilization of Coatings, ed.R. H.Winslow and S. P.Pappas. American Chemical Society Symposium Series151:183–96. Washington D.C.: ACS. Feller, R. L., and S.Raynolds. 1953. Quarterly report of the Mellon Institute, March:1–44. Feller, R. L., N.Stolow, and E. H.Jones. 1985. On picture varnishes and their solvents. Washington, D. C.: National Gallery of Art. 154–64. Frankoski, S. P., and S.Siggia. 1972. Analysis of carboxylic esters using alkali fusion reaction gas chromatography. Analytical Chemistry44(3): 507–11. Gettens, R.1935. Polymerized vinyl acetate and related compounds in the restoration of objects of art. Technical Studies4:15–27. LaFontaine, R.1979. Decreasing the yellowing rate of dammar varnish using anti-oxidants. Studies in Conservation24:14–22. Lehmann, R. A., and G. M.Brauer. 1961. Analysis of pyrolyzates of polystyrene and poly(methyl methacrylate) by gas chromatography. Analytical Chemistry33:673. Lehrle, R. S., and J. C.Robb. 1967. The quantitative study of polymer degradation by gas chromatography. Journal of Gas Chromatography5:89. Morimoto, K., and S.Suzuki. 1972. Ultraviolet irradiation of poly(alkyl acrylates) and poly(alkyl methacrylates). Journal of Applied Polymer Science16:2947–61. Radell, E. A., and H. C.Strutz. 1959. Identification of acrylate and methacrylate polymers by gas chromatography. Analytical Chemistry31:1890. Sadtler Research Laboratories. 1980. The infrared spectra atlas of monomers and polymers. Philadelphia: Sadtler Research Laboratories. 303–58, 394–412. Thomson, G.1957. Some picture varnishes. Studies in Conservation3:64–78.
Thomson, G.1963. New picture varnishes. In Recent advances in conservation. London: Butterworths. 176–84. AUTHOR INFORMATIONSUZANNE QUILLEN LOMAX received her Ph.D. in organic chemistry in 1984 from the University of Maryland, working with Patrick Mariano exploring the photochemistry of iminium salts. She then went to Northwestern University, where she performed postdoctoral research with Frederick Lewis examining intermolecular photoaddition reactions. Before beginning her work at the National Gallery, Dr. Lomax worked briefly in the Office of Toxic Substances of the Environmental Protection Agency. She has been at the National Gallery Science Department since February 1986, investigating the identification and aging behavior of artists' materials. Address: National Gallery of Art, Washington, D.C. 20565. SARAH L. FISHER graduated from Wellesley College with a B.A. in art history in 1967. The majority of her conservation training was at the Swiss Institute for Art Research in Zurich under Dr. Thomas Brachert, with additional training in other Swiss cantons and Brussels, Florence, and Amsterdam. Following jobs at the Swiss Institute, the Intermuseum Laboratory in Oberlin, Ohio, and the Balboa Art Conservation Institute in San Diego, she has worked at the National Gallery of Art in Washington, D.C., since 1981, where she is now head of painting conservation. She has published on Rubens' and Watteau's painting methods and lectured on the materials and techniques of many Old Master painters. Address: National Gallery of Art, Washington, D.C. 20565.
 Section Index Section Index |