FUNGICIDAL EFFICACY OF SELECTED CHEMICALS IN THYMOL CABINETSRALPH A. GUSTAFSON, INGRID R. MODARESI, GEORGIA V. HAMPTON, RONALD J. CHEPESIUK, & GLORIA A. KELLEY
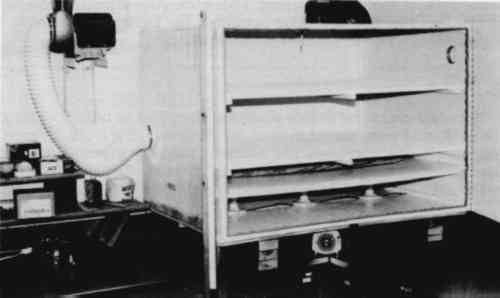
ABSTRACT—The fungicidal efficacy of eight chemicals for possible use in thymol cabinets was investigated. Of the eight, only thymol and paraformaldehyde exhibited fungicidal activity against mold cultures. None of the eight chemicals were effective in killing indigenous mold on mildewed books. 1 INTRODUCTIONMOLD (MILDEW) growth on library and archival holdings can become a significant problem in facilities that are unable to maintain indoor climatic conditions within ranges that will inhibit mold growth. Dormant mold propagules (spores and hyphal fragments) are present in the library environment in significant numbers (Burge et al. 1980; Parker 1987) and, given proper substrate (cellulose) and environment (warm temperatures and high humidity), each will germinate and establish a growing mold colony within a few days. Each of these colonies will produce millions of new propagules, which will repeat the cycle. Controlling this explosive rate of growth is a major concern to the conservator, as many of these molds (mildew molds) are cellulolytic and cause serious biodeterioration of paper. In addition to the physical damage caused by molds, many types of mold spores will cause allergic reactions in sensitive people. Burge et al. (1980), however, investigated the possible role of fungi as allergens in the University of Michigan libraries and concluded that fungal spores were not the source of the observed respiratory complaints associated with library use as measured spore counts were low and there was no distinctive library mycoflora. The growth of molds on library holdings can be prevented by carefully regulating the indoor environment of the facility. Maintaining the temperature between 60� and 65�F (15�–18�C) and keeping the RH below 60% will inhibit mold growth (Middleton 1977; Strassberg 1978). Further control could be achieved by reducing the number of mold propagules in the library environment (Middleton 1977). Such reduction would also lessen the allergen potential in the facility (Burge et al. 1980). Techniques used to reduce the number of mold propagules range from simple wiping to complete sterilization of the library holdings. Prior to the 1984 ruling by the Occupational Safety and Health Administration reducing the ethylene oxide permissible exposure level (PEL) to 1 ppm and the action level to 0.5 ppm (Art Hazards News 1984), ethylene oxide sterilizers were the preferred fumigation system for killing molds and mold spores on such materials. Since many facilities with ethylene oxide sterilizers cannot meet these reduced OSHA limits without expensive retrofitting, they have discontinued use of ethylene oxide. This situation has rekindled In principle, thymol crystals when gently heated in these cabinets generate thymol vapors to fumigate the materials placed in the cabinet (Byers 1983; Haines and Kohler 1986; Middleton 1977; Nagin and McCann 1983). In a reprinted edition of the 1982 Center for Occupational Hazards data sheet by Nagin and McCann (1983, 121) a footnote was added stating that Robert McComb of the Library of Congress suggested substituting ortho-phenylphenol for thymol “in every application” because it is “less toxic and more effective than thymol.” Subsequent to this, several agencies in South Carolina, including the State Archives, built “thymol cabinets” and started using ortho-phenylphenol (personal communication). In addition to thymol and ortho-phenylphenol, other chemicals have been considered for use in controlling mold growth on library materials, and reviews by Strassberg (1978) and Baynes-Cope (1972) are available. Throughout the literature on mildew control for library materials, there is a paucity of experimental data on the effectiveness of chemicals when used as fumigants in thymol cabinets. Kowalik (1980, 1984) conducted extensive evaluations of several chemicals for fungicidal activity, but his procedures involved direct contact between the chemical and the mold rather than gaseous fumigation systems. Others (Block 1951; Hetherington 1945; Htar 1970; Kaye 1976; Shumard 1953) tested a variety of chemicals, but again, none involved the use of thymol cabinets. The two reports using thymol cabinets give contradictory results. Florian and Byers (cited in Byers 1983) concluded that thymol was fungicidal when used under their conditions. Their research was not published, but Florian provided a copy of the internal report to the authors. Florian and Byers inoculated filter papers with fungal propagules and then exposed the dried filter papers to thymol fumes in a thymol cabinet. Their treatment cycle was 48 hours long and included two 3-hour heating periods using 20-W bulbs as the heat source. Following treatment, they transferred the filter papers to Sabouraud dextrose agar plates to assess viability. Recently, Florian referred to her study (1986), but no data were provided. Haines and Kohler (1986) reported that thymol and ortho-phenylphenol were not completely fungistatic or fungicidal in their thymol cabinets. The absence of sufficient experimental data in the literature supporting contentions about the effectiveness of various chemicals makes makes it difficult, if not impossible, to select an effective system for book fumigation. The need for an effective fumigation system at Dacus Library, Winthrop College, prompted us to undertake an evaluative study of several chemicals, using appropriate controls and procedures, to determine their potential for use in thymol cabinets. 2 MATERIALS AND METHODS2.1 CABINET CONSTRUCTIONTWO IDENTICAL thymol cabinets (fig. 1) were constructed with �-in exterior-grade plywood following published specifications (Byers 1983; Nagan and McCann 1983). All joints were sealed with silicon caulking, and the interiors were painted with two coats of epoxy paint (Byers 1983). All doors and venting apertures were sealed with rubber gasket material. Two removable slat shelves were fitted above a solid �-in hardboard divider separating the fumigation chamber from the heat source. Three 10-cm diameter holes were cut into this divider, one above each light bulb, to accommodate 15-cm diameter watch glasses. The heat sources in each cabinet were three 25-W or 40-W Philips long-life light bulbs. One cabinet was vented to the outside through a building exhaust
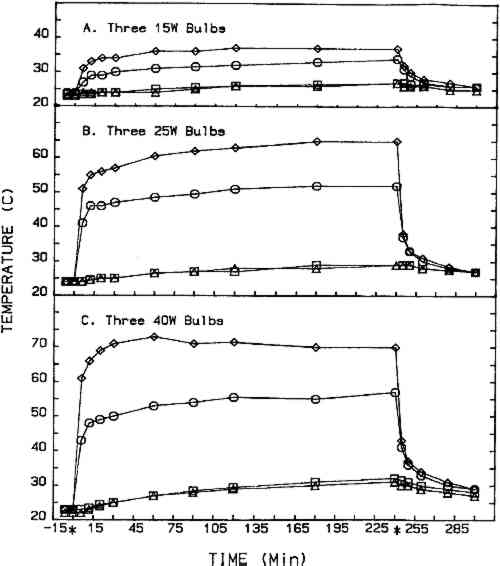
2.2 CHEMICALSThe eight chemicals evaluated in this study are described in table 1. Five grams of the chemical to be evaluated were placed in each watch glass (15 g total) for each fumigation cycle. Two chemicals, the parabens, are cosmetic industry preservatives, and their inclusion in this study was suggested by a cosmetic chemist. The remaining six chemicals are considered to have fungicidal activity (Windholz 1983). TABLE 1 Chemicals Evaluated 2.3 TEST ORGANISMSFour molds isolated from books in the Archives of Dacus Library were used throughout this study as the test organisms. They were Aspergillus niger, Penicillium sp. Cladosporium sp., and Emericella nidulans. The first three are Deuteromycetes and produce only asexual spores (conidia), and the last is an Ascomycete that produces both conidia and more resistant ascospores (sexual reproduction). 2.4 TREATMENT CYCLEEach fumigation cycle lasted 96 hours, with the timer-operated heat source (three light bulbs) on for the first 4 hours of each 24-hour segment. At the end of the 96-hour cycle, the treatment cabinet was exhausted to the atmosphere for 30 minutes prior to opening the doors. Treatment cycles for each chemical, except salicylic acid, were repeated at least once using 25-W bulbs, and one trial was completed for four chemicals using 40-W bulbs. Fresh cultures and new books were prepared for each treatment cycle. The control cabinet was fitted with four temperature probes: one directly beneath the center watch glass, one in the center watch glass, one in the center of the cabinet, and one at the top center of the cabinet. Cabinet temperatures during the 4-hour heating period for l5-W, 25-W, and 40-W bulbs are shown in figure 2. The control incubator was maintained at 25�C.
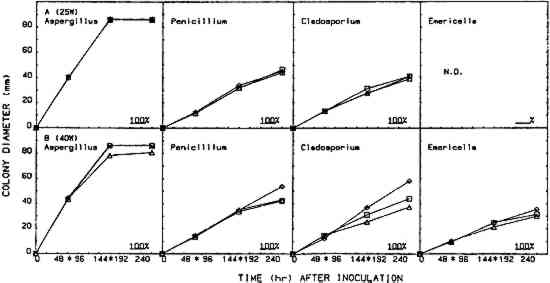
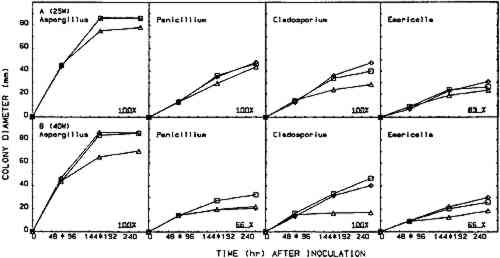
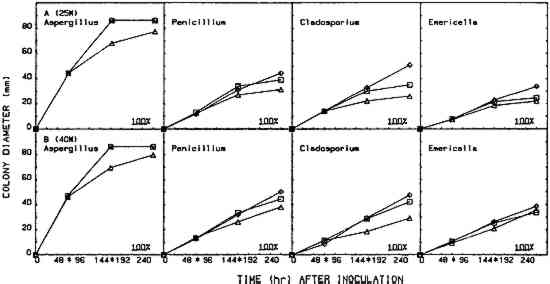
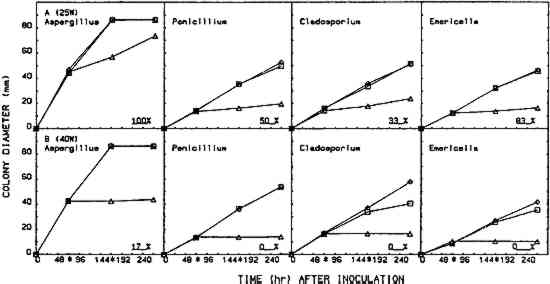
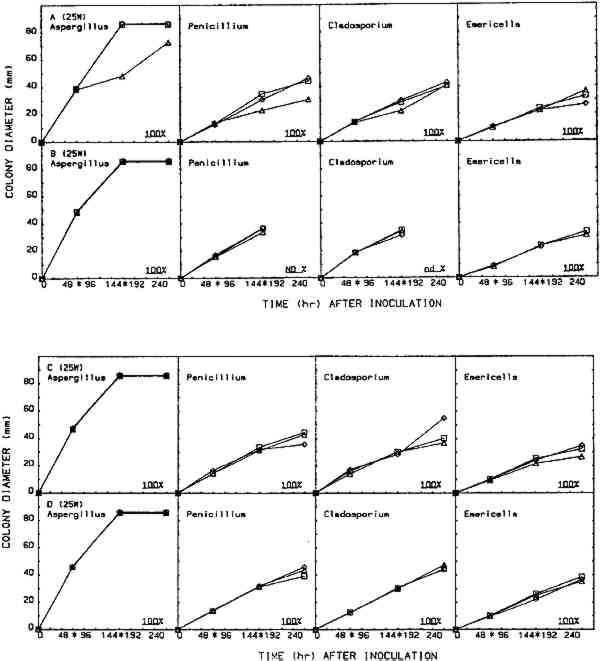
2.5 EFFICACY TESTINGThree procedures were designed to measure the fungistatic-fungicidal activity of each chemical. The first, colonial growth rate, measured the ability of each chemical to inhibit growth (fungistatic) or kill the mold (fungicidal); the second, spore germination inhibition, measured the chemical's ability to prevent germination of mold propagules; and the third evaluated the chemical's ability to kill indigenous molds on mildewed books. 2.5.1 Colonial Growth ExperimentsCultures of each mold for these experiments were spot-inoculated on Sabouraud dextrose agar plates 72 hours prior to the start of each treatment cycle. The inoculum 2.5.2 Spore Germination ExperimentsImmediately prior to the start of a treatment cycle, O.1 ml of a sterile phosphate buffer spore suspension of each organism was spread-plated on Sabouraud dextrose agar plates, and the lids on the treatment cabinet and control cabinet plates were replaced with sterile drilled lids. Five replicate plates of each organism were placed, inverted, in the treatment cabinet and control cabinet. Three replicate plates of each organism were placed in the control incubator. At the end of the 96-hour treatment cycle, the drilled lids were replaced with sterile intact lids, the plates were evaluated for growth or no growth, and all plates were then placed in the control incubator for 96 hours of post-treatment incubation. Following post-treatment incubation the plates were again evaluated for growth or no growth, and all control incubator, treatment cabinet, and control cabinet spore germination plates were subcultured onto fresh Sabouraud dextrose agar plates for viability determinations. Viability plates were evaluated for growth after 72 hours of incubation in the control incubator. 2.5.3 Book Sampling ExperimentsThese experiments involved two sampling procedures—agar overlays and swabbing. The Sabouraud dextrose agar overlay procedure was started 72 hours prior to the start of a treatment cycle. A sterile Petri dish bottom with an 8-mm diameter hole drilled in the center was inverted and taped to the top half of the front cover of each book. Fifteen ml of molten, tempered (46�C) Sabouraud dextrose sugar were pipetted onto the book cover within the dish. The hole was taped closed, and, after the agar solidified, the books were incubated in the control incubator at 25�C for 72 hours. Prior to starting the treatment cycle, the overlays were assessed for growth or no growth and the agar overlays removed from the books. The swab procedure was conducted immediately prior to starting the treatment cycle. Each book was sampled by swabbing a sterile, phosphate buffer moistened cotton-tip swab over a 9 cm2 area of the front, top left-hand corner of the book adjacent to the spine. After swabbing, the swab was used to inoculate the entire surface of a Sabouraud dextrose agar plate. Swab plates were incubated for 72 hours in the control incubator and then assessed for growth or no growth. Five of the sampled books 3 RESULTS3.1 COLONIAL GROWTH EXPERIMENTSOF THE eight chemicals evaluated in this study using the 4-hour heating period with 25-W bulbs, only thymol (fig. 4A) and paraformaldehyde (fig. 6A) showed limited fungicidal activity. Of the remaining six chemicals, two showed some fungistasis (figs. 5A, 7A) and four were ineffective (figs. 3A, 7B–D). The activity of a specific chemical varied between the four organisms included in this study. For example, thymol had little effect on Penicillium, was somewhat fungistatic to Aspergillus and Cladosporium, and was partially fungicidal to Emericella (fig. 4A).
The use of 40-W bulbs and a 4-hour heating period increased the fungicidal activity of thymol and paraformaldehyde (figs. 4B and 6B) but did not increase the activity of paradichlorobenzene (fig. 5B) and ortho-phenylphenol (fig. 5B). Again differences in sensitivity can be observed between the four organisms. None of the chemicals evaluated effected a 100% kill against all the organisms used in these experiments. 3.2 SPORE GERMINATION EXPERIMENTSThe results of these experiments (treatment cabinet only) are shown in tables 2 and 3. The results from the control cabinet and the control incubator were all 100% germination and l00% viability (data not shown). Only paraformaldehyde prevented spore germination in the experiments using the 25-W bulbs. Paraformaldehyde and, to a limited extent, thymol inhibited spore germination when 40-W bulbs were used. Once again, differences in sensitivity are apparent. TABLE 2 Percentage of Spore Gemination During Treatment Cycle (25-W Bulb, 4-Hour Heating Period)a TABLE 3 Percentage of Spore Germination during Treatment Cycle (40-W Bulbs, 4-Hour Hearing Period)a 3.3 BOOK SAMPLING EXPERIMENTSThe results of these experiments are shown in tables 4 and 5. None of the chemicals evaluated exhibited any appreciable effect on the indigenous molds on the books in those experiments utilizing 25-W bulbs. When 40-W bulbs were used, paraformaldehyde was partially effective in reducing the viable molds but did not effect a 100% kill on all books. TABLE 4 Percentage of Books Showing Growtha (25-W Bulbs, 4-Hour Heating Period) TABLE 5 Percentage of Books Showing Growtha (40-W Bulbs, 4-hour Heating Period) 4 DISCUSSIONNONE OF the eight chemicals evaluated in this study show much promise as a chemical fumigant for use in a thymol cabinet. Seven of the eight showed little or no fungicidal activity, and the only fungicidal one (paraformaldehyde) is too hazardous to consider as an alternative to ethylene oxide. Paraformaldehyde is a polymer of formaldehyde and, when heated produces formaldehyde gas. Formaldehyde has been identified as a mutagen and a carcinogen, and the current exposure level standard is 3 ppm (NIOSH 1987). Although thymol exhibited some fungicidal activity against the cultures, it was completely ineffective in killing molds on books. Ortho-phenylphenol showed no fungicidal The controversy over the use of ortho-phenylphenol in thymol cabinets (Haines and Kohler 1986; Nagin and McCann 1983; Florian 1986) stems from a footnote that was added to the authorized reprinting in Ritzenthaler (Nagin and McCann 1983) of the 1982 Center for Occupational Hazards data sheet. Although Nagin and McCann do not suggest using ortho-phenylphenol in thymol cabinets, the added footnote states that ortho-phenylphenol “should be substituted for thymol in every application” (Nagin and McCann 1983, 121). Our work and the work of Haines and Kohler (1986) raise questions about the effectiveness of thymol as a fumigant. Neither the results included in this report nor our preliminary culture results with an 8-hour treating period (data not included) demonstrate that thymol is completely fungicidal against the four organisms included in this study. Haines and Kohler (1986) also report that thymol is not completely fungistatic or fungicidal. Furthermore, our results using books showed no thymol fungicidal activity at all against the indigenous molds on the books. Nagin and McCann (1983) and Byers (1983) recommend using 15–20-W bulbs as heating elements, and Byers (1983) suggests that bulbs with a higher wattage should not be used. Our temperature recordings for 15-W bulbs do not exceed 34�C in e watch glass, well below the melting point of thymol (51.5�C). In our cabinets, only bulbs of 25-W or 40-W produced temperatures in the watch glass high enough to melt thymol, and only 40-W bulbs generated temperatures high enough to melt ortho-phenylphenol (see fig. 2). Our results show that 40-W bulbs produce greater fungistatic or fungicidal activity than 25-W bulbs. In view of this it seems unlikely that 15–20-W bulbs would generate sufficient chemical vapors of thymol or ortho-phenylphenol to kill mildew molds. Although the maximum temperature reached in the watch glasses was 57�C (135�F), the temperature in the chamber where materials would be located did not exceed 32�C (90�F). This result suggests that cabinet fumigation with 40-W bulbs would not cause heat damage to the materials and might be useful if a suitable chemical could be identified. All eight chemicals included in this study pose serious health hazards. Naphthalene, paradichlorobenzene, formaldehyde (paraformaldehyde), and ortho-phenylphenol are listed as potential carcinogens (National Institute for Occupational Safety Hazards 1987; Sax 1981; Sax and Lewis 1986). Although the other four chemicals are not specifically identified as potential carcinogens, they are toxic, and excessive exposure could produce skin, eye, and possibly respiratory irritation (National Institute for Occupational Safety Hazards 1987; Sax and Lewis 1986; Windholz 1983). It is mentioned in the literature that ortho-phenylphenol is “less toxic” than thymol, and this is correct when comparing LD50 data for oral administration to rats. However, a thorough review of the NIOSH-RTECS data sheets (National Institute for Occupational Safety Hazards 1987) suggests that ortho-phenylphenol may be more hazardous due to its potential carcinogenicity. This demonstrates that a careful review of all aspects of chemical toxicity using the most up-to-date information is necessary before reaching conclusions of chemical safety. Our work has not identified a chemical that would be effective and safe for use as a fumigant in thymol cabinets. Molds are notoriously difficult to kill, particularly in the dormant, dessicated state that they would be in on books and paper. Proper maintenance of indoor temperature and humidity seems to be the most effective way of controlling mildew growth. However, environmental control is not fungicidal, and any lapse in control could easily result in rapid growth and spread of mildew molds. It is interesting to speculate about the actual need to fumigate books and papers as a means of mold (mildew) control. A simple scenario illustrates such speculation. Givens: Mold propagules are everywhere; provided the proper substrate and environment, they will germinate and produce mold colonies that develop millions of new propagules; these new propagules disseminate, and the growth cycle is repeated many times; and fumigation does not leave any inhibiting residue on the materials. Result: Mold growth everywhere! Due to the tremendous reproductive potential of molds, it may not be of value to take preventive measures other than ensuring appropriate indoor climate control (Parker 1987). However, it can be argued that a minimal physical removal of mold growth (brushing, wiping, vacuuming) will reduce the number of mold spores that could cause allergic reactions in the users of the facility. This result alone seems important enough to warrant physical removal, outdoors, of mold propagules from holdings prior to placing these items in circulation. ACKNOWLEDGEMENTSThis work was supported by a grant (CLR 804) from the Council on Library Resources, Washington, D.C. Appreciation is also extended to all the faculty members of Winthrop College who lost their personal libraries to water damage following a campus building fire. Their loss was our gain, as we had several hundred mildewed books to include in our study. Appreciation is extended to the Chemistry and Physics Department for use of computer hardware and software for preparation of figures 2–7 and to Mary Lou Florian for sharing her data with us. REFERENCESArt Hazards News. 1984. OSHA issues ethyleoide standard. Art Hazards News7(6):1–2. Baynes-Cope, A.1972. Choice of biocides for library and archival material. Proceedings of the second international biodeterioration symposium. New York: Halstead Press. 381–87. Block, S. S.1951. Experiments in mildew prevention. Modern Sanitation3(12):61–66. Burge, H. P., J. R.Boise, R.Solomon, and E.Bandera. 1980. Fungi in libraries: An aerometric survey. Mycopathologia64:67–72. Byers, B.1983. A simple and practical fumigation system. Abbey Newsletter7(4): 1–4, suppl. Florian, M. L.1986. Letter to the editor. Journal of the American Institute for Conservation25:109. Haines, J. H., and S. A.Kohler. 1986. An evaluation of ortho-phenylphenol as a fungicidal fumigant for archives and libraries. Journal of the American Institute for Conservation25:49–55.
Hetherington, D. C.1945. Mold preventive for book bindings. Science101:223. Htar, K. T.1970. A comparative study of three fungicides for preservation of books. Union of Burma Journal of Life Sciences3(1):87–89. Kaye, S.1976. New, nondamaging preventatives of biodeterioration–monochloroacetate esters of clorinated phenols. Developments in Industrial Microbiology17:159–65. Kowalik, R.1980. Microbiodeterioration of library materials. Restaurator4:99–114: 135–219. Kowalik, R.1984. Microbiodeterioration of library materials. Restaurator6:61–115. Middleton, B. C.1977. Book preservation for the librarian. In Preservation of paper and textiles of historic and artistic value, ed.J. C.Williams. Advances in Chemistry 164. Washington, D.C.: American Chemical Society. 3–23. Nagin, D., and M.McCann. 1983. Thymol and o-phenylpenol: Safe work practices. In Archives and manuscripts: Conservation, ed.M. L.Ritzenthaler. Chicago: Society of American Archivists. 121–24. NIOSH. 1987. Registry of toxic effects of chemical substances. Washington, D.C.: National Institute for Occupational Safety Hazards. Parker, T. A.1987. Integrated pest management for libraries. In Preservation of library materials, ed.M. A.Smith. New York: K. G. Saur. 103–23. Sax, N. I.1981. Cancer-causing chemicals. New York: Van Nostrand Reinhold. Sax, N. I., and R. J.Lewis, Sr.1986. Rapid guide to hazardous chemicals in the workplace. New York: Van Nostrand Reinhold. Shumard, R. S.1953. Mildew control: Possibilities of paradichlorobenzene as an aid in preventing damage by mildew. Soap and Sanitary Chemicals29: 150–153, 171. Strassberg, R.1978. The use of fumigants in archival repositories. American Archivist41(1):25–36. Windholz, M., ed.1983. The Merck index. 10th ed.Rahway, N.J.: Merck. AUTHOR INFORMATIONRALPH A. GUSTAFSON is a professor of biology, specializing in microbiology and mycology, in the Biology Department of Winthrop College, Rock Hill, South Carolina. He holds B.S. and M.Ed. degrees from Colorado State University and a Ph.D. degree in mycology from the University of Texas—Austin. He has published scientific articles in the Journal of Bacteriology, Mycologia, Journal of Environmental Health, and ASM News and has completed several consulting projects evaluating the effectiveness of microbiocides in paper coatings for the manufacturer. Address: Biology Department, Winthrop College, Rock Hill, South Carolina 29733. INGRID R. MODARESI is a high school science teacher. She obtained her B.S. in biology in 1985 and her M.A.T. in secondary education in 1988 from Winthrop College in Rock Hill, South Carolina. Her special area of interest is environmental biology. GEORGIA V. HAMPTON earned a B.S. degree in biology from Winthrop College in 1986 and a M.S. in biology the following year. She taught biology at Rock Hill High School and at Orangeburg-Calhoun Technical College. She is presently department head of Natural Sciences at Orangeburg-Calhoun Technical College. RONALD J. CHEPESIUK is head of the Archives and Special Collections at the Dacus Library, Winthrop College, Rock Hill, South Carolina. He holds a M.L.S. from Atlanta University and a D.A.S. from the National University of Ireland, University College, Dublin. His professional articles have appeared in the American Archivist, Provance, New Library Scene, Wilson Library Bulletin, Library Journal, and American Libraries. GLORIA A. KELLEY is head of Technical Services at Ida Jane Dacus Library, Winthrop College, Rock Hill, South Carolina. She holds a master of science degree in library service from Atlanta University, Atlanta, Georgia and has extensive training in preservation. She attended Columbia University Rare Book School in summer 1989. She serves as preservation consultant for the Palmetto Archives, Libraries and Museum Council on Preservation in South Carolina and serves as consultant to libraries in the area of basic book repair techniques and disaster planning.
 Section Index Section Index |