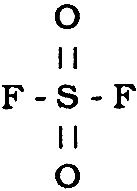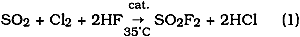SULFURYL FLUORIDE (VIKANE): A REVIEW OF ITS USE AS A FUMIGANTMICHELE R. DERRICK, HELEN D. BURGESS, MARY T. BAKER, & NANCY E. BINNIE
3 CHEMICAL REACTIVITYSulfuryl fluoride (SO2F2) is a distorted tetrahedral molecule represented by the structure:
Dow has patented the process for its production (Cook and Gustafson 1978) from an anhydrous gaseous mixture of sulfur dioxide, chlorine, and hydrofluoric acid in the presence of a charcoal catalyst heated to 35�C:
This process results in a product with a purity of greater than 99%. The impurities are composed of residual starting materials and byproducts in concentrations of 2000 ppm or less in the liquid Vikane (table 2). While the concentrations of these low-level impurities (HF, HCl, Cl2, SO2 and ethylene dichloride) may not be high enough after dispersal to cause observable changes to materials, their presence is important since most of them are highly reactive. Conservators must also be concerned with the possibility of cumulative fumigation effects, since museum collections are stored over long periods and may be fumigated repeatedly. Thus, as a precaution, the acidic impurities (HF and HCl) may be removed by passing the gaseous Vikane through a filter tube filled with marble chips (Derrick 1989). Table 2 Vikane Analysis of Cylinder #870303 (Lot #872) Used for Field Fumigations in Joint GCI, CAL, CCI Research Project The solubility of Vikane in water is very low; a saturated aqueous solution contains 750 ppm at 25�C and 1 atm (Meikle and Stewart 1962). Cady and Sudhindra (1974) found that
In a basic solution of pH greater than 7.5, nucleophilic displacement of fluorine by ammonia, phenyl, or cyanide groups occurs readily. This base-catalyzed hydrolysis may occur at high humidities in some high-pH materials such as basic pigments and paper with alkaline reserve, and this possibility should be thoroughly examined. A study using the specially prepared radioactive isotope-labeled fumigant (S35) for the determination of the distribution of reaction products in graham flour found indications that the nitrogen in some proteins and amino acids combines readily with sulfuryl fluoride to produce N-fluorosulfonyl derivatives (Meikle 1964). The radioactive S35 was bound to the protein fraction, and it was determined that the sulfuryl fluoride selectively reacted with the amino groups rather than the carboxyl groups. Furthermore, it was found that fluoride ion, a decomposition product of Vikane, is present as an inorganic residue in wheat flour. An initial Vikane study by Dow using radioisotopic labeling (S35) found that household materials (food, rubber, sugar, wood, cotton, paper, wool, nylon, and various synthetic materials) exposed to sulfuryl fluoride had smaller residues than those exposed to methyl bromide (Meikle and Stewart 1962). In fact, the only materials that retained any significant amounts of residues from sulfuryl fluoride exposure were proteinaceous food products, such as beef and cheese. A recent study measured fluoride and sulfate residues in unprotected foodstuffs, fumigated at 360 g/m3 for 20 hours (approximately 100 times the dosage used for drywood termites) and found high (500-2000 ppm) levels in dry milk and beef; moderate (100-500 ppm) levels in dog food and flour; low (10-100 ppm) levels in Vikane is nonflammable and stable under normal conditions. However, Vikane does decompose when exposed to high temperatures (>400�C) to form a toxic, reactive gas consisting of hydrofluoric acid and sulfur dioxide (Dow Chemical Co. 1982). These reactive decomposition products may affect materials such as metals, glass, ceramic tiles, china, dyes, and pigments. Thus, prior to any structural fumigation all heat sources, such as pilot lights, electric heater elements, or automatic switches, should be turned off or disconnected. |