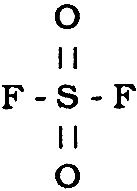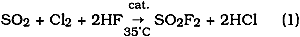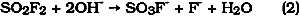SULFURYL FLUORIDE (VIKANE): A REVIEW OF ITS USE AS A FUMIGANTMICHELE R. DERRICK, HELEN D. BURGESS, MARY T. BAKER, & NANCY E. BINNIE
ABSTRACT—Sulfuryl fluoride, sold commercially as a fumigant by Dow Chemical Company under its trademark Vikane, is currently being examined for use in museums and historic structures as a pest control agent. While little to no visible damage to materials is noted when Vikane is properly used, the selection criteria for a museum fumigant must be founded on an in-depth analysis of its possible effect on the physical and chemical properties of exposed artifacts. This article forms a basis for the evaluation of sulfuryl fluoride by providing a comprehensive review on Vikane, including information regarding the uses, chemical reactivity, physical properties, efficacy, and toxicity of sulfuryl fluoride. 1 INTRODUCTIONBY DEFINITION, a fumigant is a toxic, gas-phase, chemical agent that is lethal to pests, insects and/or micro-organisms (Hawley 1987). This implies several aspects of the fumigant and its properties:
A comprehensive assessment of the above aspects of a fumigant, along with its methods of use, aids in its selection as an option for the eradication of pests from an object or building and should be made prior to its use. An ideal fumigant for insect pests would penetrate rapidly into infested areas, effectively kill all stages of development of the organism, remain nonreactive to the material being fumigated, and disappear completely from the fumigated materials upon aeration. This degree of selectivity is rarely the case; in fact, most fumigants that have been or are currently in use by museums do alter materials to varying degrees (table 1). For example, methyl bromide reacts with sulfur-containing materials such as furs, hair, feathers, and leather goods producing a malodorous product (Dow Chemical Co. 1957). Phosphine, hydrogen cyanide, carbon disulfide, and dichlorvos react with metals and some pigments (Monro 1972), and thus any material containing a metal, such as a weighted fabric or a mordanted dye, may be susceptible. Ethylene oxide has been found to react with cellulose Table 1 Some Fumigants Used in Museums and Their Reactivity with Various Materials Faced with these problems, conservators have been seeking alternative methods to fumigation. Approaches have been made to bypass toxic chemicals in favor of methods that prevent infestations, such as integrated pest control management programs (Parker 1987), or by using less problematic methods of killing pests, such as freezing (Florian 1987; Nesheim 1984) and oxygen deprivation using carbon dioxide (Sanders 1987) or nitrogen (Ali Niazee 1972; Valentin and Preusser 1989). However, these methods are still in the process of being evaluated and are suitable mainly for movable artifacts and not for the treatment of buildings, with or without their contents. Thus, some cases may require One possibility is sulfuryl fluoride, a fumigant manufactured by Dow Chemical Company and sold under the name of Vikane. In recent field studies and structural fumigations using Vikane, little or no damage to the fumigated material has been observed. Therefore, sulfuryl fluoride has been selected for an in-depth study to determine what unobservable effects, if any, Vikane may have on materials commonly found in museums. This review article is the first step in a program developed to provide conservators with specific, detailed information on the use of Vikane, and it forms a basis for the evaluation of sulfuryl fluoride. An extensive study on the effects of Vikane on various materials and its suitability for use in museum chambers and structural fumigations is being conducted by the Getty Conservation Institute (GCI) in conjunction with the Canadian Conservation Institute (CCI) and the Smithsonian Institution's Conservation Analytical Laboratory (CAL). Also, through a contract with GCI, a group at the University of Florida is reevaluating fumigant concentrations recommended for structures to determine the lowest levels that may be used in controlled museum applications. While some preliminary results of these projects will be discussed in this paper, the conclusions and supporting data regarding the use of sulfuryl fluoride on museum materials will be published in subsequent articles. The annotated bibliography of the Vikane literature, developed during this study, is available upon request from the Scientific Department, GCI. 2 VIKANE GAS FUMIGANTVIKANE WAS developed by Dow Chemical in the 1950s specifically for the control of drywood termites that are typically found in warm climates, such as the southern United States (Dow Chemical Co. 1982). It has since been widely used as a structural fumigant for homes, buildings, construction materials, furnishings, and vehicles for a variety of destructive pests. Studies conducted on Vikane show that it has several advantages, including easy dispersal into a structure, rapid penetration into materials, formation of almost no residues, and ready dissipation after aeration (Meikle and Stewart 1962). Although Vikane is a gas at atmospheric conditions, it is stored in cylinders as a liquid under its own pressure. For dispersal into a room or chamber, the liquid is released through an application hose or tube toward distribution fans. Volatilization of the liquid occurs rapidly after it is released from the cylinder. This is important, since liquid Vikane is more harmful to objects and materials than the gas and can tarnish metals and damage ceramic tiles. Some precautions, such as fumigating only when the temperature is above 12�C and moving objects out of the direct application path, should be taken to ensure that all the liquid Vikane is volatilized prior to reaching any object. To eliminate this possibility altogether, a heat exchanger may be used to convert the liquid to a gas prior to dispersal. Since Vikane is 3.5 times heavier than air, the air must be circulated at the beginning of the fumigation period to ensure rapid, uniform distribution throughout the fumigation area. After fumigation is complete and the sulfuryl fluoride is released from the building or exposure chamber, the gas moves outward swiftly from the area of containment. From Dow's calculations, it would take less than two hours for 4 g Vikane/m3 in air (962 ppm, a typical dosage for drywood termites [4 oz/1000 ft3]) to be reduced to less than 9 ppm in a house with doors and windows open and a moderate wind (Dow Chemical Co. 1982). The concentration of sulfuryl fluoride can be monitored during fumigation using a Fumiscope, a thermoconductivity unit that reads from 0.62–1000 g/m3(150–240,000
∗ Fumiscope. R.K. Hassler Company, P.O. Box 1968, Mammoth Lakes, CA 93546. The units can be purchased through any Dow distributor. Interscan model GF 1900. Interscan corporation, 21700 Nordoff Street, P.O. Box 2496, Chatsworth, CA 91311. The wilks Miran 101 analyzer. Foxboro Analytical, Wilks Infrared Center, 120 water Street, P.O. Box. 449, South Norwalk, CT 06858. Vikane is a Restricted Use fumigant to be purchased and applied only by licensed fumigators who usually carry special types of insurance for such applications. It is labeled for use in museum chambers by licensed fumigators, either on staff or on a contract basis. Vikane has been used successfully to fumigate some historic structures and their collections, such as the Hearst Castle in California (Pest Control 1977) and the Flagler Mansion in Florida (Moon 1981). The United States, which uses Vikane for 50% of all structural fumigations, and Japan are presently the only countries in which Vikane is registered for use. 3 CHEMICAL REACTIVITYSulfuryl fluoride (SO2F2) is a distorted tetrahedral molecule represented by the structure:
Dow has patented the process for its production (Cook and Gustafson 1978) from an anhydrous gaseous mixture of sulfur dioxide, chlorine, and hydrofluoric acid in the presence of a charcoal catalyst heated to 35�C:
This process results in a product with a purity of greater than 99%. The impurities are composed of residual starting materials and byproducts in concentrations of 2000 ppm or less in the liquid Vikane (table 2). While the concentrations of these low-level impurities (HF, HCl, Cl2, SO2 and ethylene dichloride) may not be high enough after dispersal to cause observable changes to materials, their presence is important since most of them are highly reactive. Conservators must also be concerned with the possibility of cumulative fumigation effects, since museum collections are stored over long periods and may be fumigated repeatedly. Thus, as a precaution, the acidic impurities (HF and HCl) may be removed by passing the gaseous Vikane through a filter tube filled with marble chips (Derrick 1989). Table 2 Vikane Analysis of Cylinder #870303 (Lot #872) Used for Field Fumigations in Joint GCI, CAL, CCI Research Project The solubility of Vikane in water is very low; a saturated aqueous solution contains 750 ppm at 25�C and 1 atm (Meikle and Stewart 1962). Cady and Sudhindra (1974) found that
In a basic solution of pH greater than 7.5, nucleophilic displacement of fluorine by ammonia, phenyl, or cyanide groups occurs readily. This base-catalyzed hydrolysis may occur at high humidities in some high-pH materials such as basic pigments and paper with alkaline reserve, and this possibility should be thoroughly examined. A study using the specially prepared radioactive isotope-labeled fumigant (S35) for the determination of the distribution of reaction products in graham flour found indications that the nitrogen in some proteins and amino acids combines readily with sulfuryl fluoride to produce N-fluorosulfonyl derivatives (Meikle 1964). The radioactive S35 was bound to the protein fraction, and it was determined that the sulfuryl fluoride selectively reacted with the amino groups rather than the carboxyl groups. Furthermore, it was found that fluoride ion, a decomposition product of Vikane, is present as an inorganic residue in wheat flour. An initial Vikane study by Dow using radioisotopic labeling (S35) found that household materials (food, rubber, sugar, wood, cotton, paper, wool, nylon, and various synthetic materials) exposed to sulfuryl fluoride had smaller residues than those exposed to methyl bromide (Meikle and Stewart 1962). In fact, the only materials that retained any significant amounts of residues from sulfuryl fluoride exposure were proteinaceous food products, such as beef and cheese. A recent study measured fluoride and sulfate residues in unprotected foodstuffs, fumigated at 360 g/m3 for 20 hours (approximately 100 times the dosage used for drywood termites) and found high (500-2000 ppm) levels in dry milk and beef; moderate (100-500 ppm) levels in dog food and flour; low (10-100 ppm) levels in Vikane is nonflammable and stable under normal conditions. However, Vikane does decompose when exposed to high temperatures (>400�C) to form a toxic, reactive gas consisting of hydrofluoric acid and sulfur dioxide (Dow Chemical Co. 1982). These reactive decomposition products may affect materials such as metals, glass, ceramic tiles, china, dyes, and pigments. Thus, prior to any structural fumigation all heat sources, such as pilot lights, electric heater elements, or automatic switches, should be turned off or disconnected. 4 PHYSICAL PROPERTIESFUMIGANTS ARE used to control insect infestations in inaccessible places such as within wood, walls, or other hiding places. In order to kill insects, an adequate concentration of the fumigant must penetrate all types of materials rapidly and remain in the presence of the insect life stages for a sufficient amount of time, ideally without being absorbed by the material. Vikane is effective in this regard, exhibiting high penetration rates and low absorption characteristics (table 3). Table 3 Physical Properties of Sulfuryl Fluoride At room temperature, sulfuryl fluoride has a vapor pressure of 13,400 mm Hg, more than eight times greater than that of methyl bromide. The high vapor pressure, low substrate reactivity, and high diffusion rate of Vikane provide rapid penetration of toxic amounts into the pest sites. Using kill efficiency studies Kenaga (1957) demonstrated that Vikane penetrates materials and soil much better than methyl bromide. (“Kill efficiency” describes the percent mortality of the insects being examined, and it is used to A recent study looked at the desorption rates of Vikane from typical foodstuffs. Osbrink et al. (1987) found vegetable oil desorbed the most; apple, dog food, cake, and flour fell in the middle; and milk, beef, and acetaminophen desorbed the least. Comparison of the desorption amounts to the residual fluoride and sulfate found in Scheffrahn et al. (1989) indicates materials that react with Vikane have correspondingly lower desorption amounts. However, neither of these studies measured the amount of Vikane that was absorbed in a material initially; thus, a substance, such as acetaminophen, can have low desorption and low residues because of its low physical and chemical reactivity, respectively. Another study on Vikane desorption rates for household objects found measurable amounts (>0.1 ppb) of Vikane desorbing 26 hours after fumigation (at 36 g/m3 for 20 hours, approximately 10 times the dosage used for drywood termites) in concrete blocks, gypsum/cardboard drywall, fiberglass insulation, polystyrene insulation, wood, topsoil, carpet padding, polyester cushion fiber, wool fabric, cotton fabric, baseball glove, latex baby bottle nipples, and a toy soldier (Scheffrahn et al. 1987). Forty days after treatment, ppb levels of Vikane were still desorbing from the polyester cushion, the polystyrene insulation, the wool fabric, and the toy. 5 EFFICACYTHE BIOCIDAL effect of a fumigant depends not only on the concentration that reaches the specific insect, but also on the time that the fumigant is present and the physiological response of the species. Certain stages of insect development, such as the egg, have slow metabolic processes, which require a longer exposure time (Kenaga 1957). Thus, most efficacy studies report the integrated concentration (concentration � time) required to kill a given species at a specific developmental stage. Temperature and humidity also affect the efficacy of a fumigant. At several exposure lengths and temperatures, sulfuryl fluoride was found to be more toxic than the same concentration of methyl bromide for beetles (Kenaga 1957). Efficacy studies have determined the concentrations of Vikane required to kill at least 95% (LD95) of the adults for various species in a 16-hour period (table 4). The LD95 on all the life stages of the insects tested, except for the egg stage, are less than Dow's recommended dosages, which are calculated based on the conditions for the fumigation (Dow Chemical Co. 1982). Thus, the recommended concentrations for fumigation in a contained space should be more than sufficient to kill most pests. In fact, much lower concentrations may be used to kill some insects and therefore minimize the amount of Vikane available for deleterious reactions to materials. The University of Florida has conducted efficacy measurements to determine the minimum dosage required for several beetle species, commonly found in museums, at each stage of their development (Su 1990). In addition to the pests listed in table 4, Vikane has been used successfully on postembryonic forms of the following species: Oriental and brown-banded cockroaches, death watch and powder post beetles, clothes moths, old house borers, fleas, rodents, ants, bees, wasps, spiders, millipedes, bedbugs, silverfish, springtails, earwigs, fire brats, and book lice (Dow Chemical Co. 1982); southern armyworms and Mexican bean beetles Table 4 Efficacy Studies on Vikane for Selected Insects Beetle eggs can require as much as 76 g Vikane/m3 air in order to kill them (Kenaga 1957). One study found that it took at least 24 hours for the fumigant to penetrate the shell of some termite eggs (Outram 1967a). Outram (1967b) suggested that this resistance is due mainly to the impermeability of the egg shell to the fumigant, with most of it being chemically held by the outer protein layer of the egg shell as well as the embryonic membranes. Sulfuryl fluoride may have multiple biochemical effects on a target species. One study, on insect eggs of the desert locust and yellow mealworms, indicated that Vikane gas reduced the egg's oxygen uptake in addition to disturbing its phosphate balance and partially suppressing the hydrolysis of several fatty acids (Outram 1970). Another study indicated that fluoride was the primary toxin and that the glycolysis process was blocked, which inhibited the insect's mechanism to maintain a sufficient source of energy (Meikle, Stewart, and Globus 1963). Like other fumigants, Vikane has no residual pest control effects. Care must be taken that the collection is not reinfested. The microbiological effectiveness of Vikane has not previously been examined. Current work indicates that the presence of Vikane decreases the activity of some bacteria and fungi (tested on Aspergillus niger, Aspergillus flavus, Actinomyces sp., Penicillium sp., and Bacillus subtilis, cultured from proteinaceous materials), but that it does not kill the spores when applied at the concentration levels recommended for insects (Valentin 1950). In this respect, Vikane is less effective than ethylene oxide as a sterilizing agent. 6 TOXICITYVIKANE IS an odorless, colorless gas that is not irritating to the eyes or skin. This inability to cause human sensory recognition is dangerous since sulfuryl fluoride can be lethal to humans in a single exposure to normal fumigation concentrations of Vikane in air (Dow Chemical Co. 1989). For safety reasons, Dow recommends that trace amounts of chloropicrin, a highly acrid, lachrymal gas, be released in the structure prior to fumigation as a warning agent. (The effect of chloropicrin on museum materials is not known. Thus, when possible, artifacts should be treated in a safe manner that does not require the presence of chloropicrin.) Since Vikane, in liquid and gaseous form, is not absorbed through the skin, the most likely route of exposure and overexposure is inhalation. The lethal dose by inhalation (LC50) for rats is in the range of 1000-4000 ppm for 1-4-hour exposures (Dow Chemical Co. 1989; Nitschke et al. 1986). Rats and rabbits exposed to 100 ppm of sulfuryl fluoride for 6 hours per day, 5 days per week for 13 weeks had mottled teeth and slightly diminished sensory functions, while animals exposed to 30 ppm exhibited no treatment effects (Mattson et al. 1988; Eisenbrandt and Nitschke 1989). Some deaths have been attributed to sulfuryl fluoride. In two cases, individuals, despite numerous warning signs, entered tented houses during the Vikane containment period. Both inhaled lethal doses of the fumigant. Autopsies revealed higher than normal levels of fluoride in both the plasma and urine (Scheuerman 1986). Another case was reported recently in Art Hazards News(McCann 1987) in which an elderly couple died after returning to their house, which had been fumigated with sulfuryl fluoride, aerated, and certified safe for reentry (Morbidity and Mortality Weekly Report 1987). Although autopsy information was lacking and the death profiles were atypical for sulfuryl fluoride, the exterminator in the case was held liable for the deaths because the recommended procedures for Vikane fumigation, such as checking the fumigant concentration with proper instrumentation prior to permitting the occupant to enter, were not followed (New York Times 1988). The recommended exposure levels are 5 ppm TLV (maximum exposure for 8 hours a day, 5 days a week) and 10 ppm STEL (maximum short term exposure limit) (Dow Chemical Co. 1989). Overexposure may cause abdominal pains, nausea, vomiting, convulsions, or chemical pneumonia. Some possible chronic effects from exposure are lung and kidney damage, central nervous system damage, and teeth and bone defects from fluorosis. Vikane was found to be teratogenic in animals only when it reached levels at which the mother was injured or killed. Serious long-term mutagenic, carcinogenic, and reproduction effects of sulfuryl fluoride are unknown. Using animals, sulfuryl fluoride was shown to be one-half to one-third as toxic as methyl bromide (Torkelson, Hoyle, and Rowe 1966). No studies have investigated the long-term exposure effects of Vikane on humans. However, one study, on the effects of methyl bromide on the neurobehavioral functions of professional fumigators, selected as a control group some fumigators who solely used 7 SUMMARYVIKANE (SULFURYL FLUORIDE) has, thus far, been an effective structural fumigant. In comparison to methyl bromide fumigant, Vikane penetrates materials better, is more toxic to pests, and leaves fewer residual compounds in materials after aeration. Little visible damage on materials of objects has been reported in fumigations with Vikane when all the Dow-recommended procedures for release of the chemical are followed. These characteristics make Vikane look promising for use as a museum fumigant. Little has been done, however, to assess thoroughly the short-term and long-term effects of using Vikane on materials that may be found in museums. Based on the information given in this paper, some materials that may be susceptible to Vikane (in gaseous or liquid form) and/or to trace levels of impurities present in the commercial fumigant are alkaline and proteinaceous materials, acid-sensitive materials, and metals. Since possible reactions may not produce visible changes, the chemical and physical properties of materials need to be examined and related to the lifetime of the object, potential repeat fumigations, and damages due to infestation. Many of these questions should be answered by studies being conducted by a joint research team (GCI, CCI, and CAL). The results, along with the efficacy information obtained from the University of Florida project, should provide sufficient information for conservators to make an informed decision on the use of Vikane as a fumigant for pest control in museums and historic structures. Results from this study will be published individually by each participating institution. ACKNOWLEDGEMENTSTHE AUTHORS would like to thank Frank Preusser (GCI), Ken Macleod (CCI), and Lambertus van Zelst (CAL) for initiating this project. The authors appreciate the information on fumigation usage and regulations provided by Thomas Parker (Pest Control Services, Inc.) and Jim Bean (Dow Chemical Co.). We are also grateful to Nieves Valentin (Instituto de Conservacion y Restauracion de Bienes Culturales, Madrid) for her help and suggestions. This work and the in-depth study of the effects of Vikane on materials in museum artifacts were sponsored by contracts from the Getty Conservation Institute to the Canadian Conservation Institute, the Smithsonian Institution's Conservation Analytical Laboratory, and the University of Florida at Ft. Lauderdale. REFERENCESAli Niazee, M. T.1972. Susceptibility of the confused and red flour beetles to anoxia produced by helium and nitrogen at various temperatures. Journal of Economic Entomology65(1):60–64. Anger, W. K., L.Moody, J.Burg, W. S.Brightwell, B. J.Taylor, J. M.Russo, N.Dickerson, J. V.Setzer, B. L.Johnson and K.Hicks. 1986. Neurobehavioral evaluation of soil and structural fumigators using methyl bromide and sulfuryl fluoride. NeuroToxicology7(3):137–56. Ballard, M., and N.Baer. 1981. Ethylene oxide fumigation: results and risk assessment. Paper presented at annual meeting of the Society of American Archivists, Washington, D.C., September 1981. Berck, B.1966. Investigations on fumigants. Occupational Health Review18(1):16–26. Bess, H. A., and K. O.Asher. 1960. Fumigation of buildings to control the drywood termite. Journal of Economic Entomology53(4): 503–10. Bockhoff, F. J., R. V.Petrella, and E. L.Pace. 1960. Thermodynamic properties of sulfuryl fluoride from 12K to its boiling point. Entropy from molecular and spectroscopic data. Journal of Chemical Physics32(3):799–804. Cady, G. H., and M.Sudhindra. 1974. Hydrolysis of sulfuryl fluoride. Inorganic Chemistry13(4):837–41. Cartwright, M., and Woolf, A. A.1977. The heat of formation of sulphuryl fluoride and the stability of fluorosulphates. Journal of Fluorine Chemistry9:495–504. Conservation Information Network, Materials Database. 1987. Vikane, MCIN record no. 908. Cook, D. M., and D. C.Gustafson. 1978. Process for preparing sulfuryl-fluoride and chlorofluoride products. United States Patent No. 4,102,987, July 25, 1978. Derrick, M.1989. Unpublished results, Getty Conservation Institute. Dow Chemical Co. 1957. Commodities unsuited for methyl bromide fumigation. Dow Fumifacts4:2. Dow Chemical Co. 1982. Vikane Fumigation Manual. Dow Chemical Co. 1989. Material safety data sheet no. 000506, January 23, 1989. Eisenbrandt, D. L., and K. D.Nitschke. 1989.Inhalation toxicity of sulfuryl fluoride in rats and rabbits. Fundamentals of Applied Toxicology12:540–57. Florian, M.1987. The effect on artifact materials of the fumigant ethylene oxide and freezing used in insect control. ICOM Committee for Conservation, 8th Triennial Meeting, Sydney, 1:199—208. Green, L., and V.Daniels. 1985. Investigation of the residues formed in the fumigation of museum objects using ethylene oxide. In Recent Advances in the Conservation and Analysis of Artifacts, comp. James Black. London: Summer School Press. 1–16 Hawley, G. G.1987. Condensed Chemical Dictionary, 10th ed. Kenaga, E. E.1957. Some biological, chemical and physical properties of sulfuryl fluoride as an insecticidal fumigant. Journal of Economic Entomology50(1):1–6. Kenjo, T.1977. Effects of insecticidal and fungicidal agents on materials of cultural properties. Scientific Papers on Japanese Antiques and Art Crafts19–20:83–87.
Marano, D.1984. Chemical fumigants in the grain handling industry. Family and Community Health7(3):76–82. Mattson, J. L., R. R.Albee, D. L.Eisenbrandt, and L. W.Chang. 1988. Subchronic neurotoxicity in rats of the structural fumigant, sulfuryl fluoride.Neurotoxicology and Teratology10(2):127–33. McCann, M.1987. Sulfuryl fluoride fumigation fatalities. Art Hazards News10(9):1. Meikle, R. W., and D.Stewart. 1962. Structural fumigants, the residue potential of sulfuryl fluoride, methyl bromide, and methane-sulfonyl fluoride in structural fumigations. Journal of Agricultural and Food Chemistry10:393–97. Meikle, R. W., D.Stewart, and O. A.Globus. 1963. Drywood termite metabolism of vikane fumigant as shown by labelled pool technique. Journal of Agricultural and Food Chemistry11:226–30 Meikle, R. W.1964. The fate of sulfuryl fluoride in wheat flour. Journal of Agricultural and Food Chemistry12:464–67. Monro, H.A.U.1972. Manual of fumigation for insect control. FAO Agricultural StudiesUNIPUB, Inc., New York. Moon, B. L.1981. Vikane gas helps save the Taj Mahal of North America. Industrial Vegetation and Pest Management13(1):12–15. Morbidity and Mortality Weekly Report. 1987. Fatalities resulting from sulfuryl fluoride exposure after home fumigation—Virginia. Morbidity and Mortality Weekly Report36 (September 18, 1987):602. Nesheim, K.1984. The Yale non–toxic method of eradicating book eating insects by deep–freezing. Restaurator6:147–54. New York Times. 1988. Pest control company fined $500,000 in death of couple. New York Times, November 18, 1988, C19. Nitschke, K. D., R. R.Albee, J. L.Mattson, and R. R.Miller. 1986. Incapacitation and treatment of rats exposed to a lethal dose of sulfuryl fluoride. Fundamentals of Applied Toxicology7:664–70. OsbrinkW.L.A., R. H.Scheffrahn, N. Y.Su, and M. K.Rust. 1987. Laboratory comparisons of sulfuryl fluoride toxicity and mean time of mortality among ten termite species (Isoptera, Hodotermitidae, Kalotermitidae, Rhinotermitidae), Journal of Economic Entomology80(5):1044–47. Osbrink, W.L.A., R. H.Scheffrahn, R. C.Hsu, and N. Y.Su. 1988. Sulfuryl fluoride residues of fumigated foods protected by polyethylene bags. Journal of Agricultural and Food Chemistry36(4):853–55. Outram, I.1967a. Factors affecting the resistance of insect eggs to sulphuryl fluoride—I: The uptake of sulphuryl-35S fluoride by insect eggs. Journal of Stored Products Research3(May 22, 1967):255–60. Outram, I.1967b. Factors affecting the resistance of insect eggs to sulphuryl fluoride—II: The distribution of sulphuryl-35S fluoride by insect eggs. Journal of Stored Products Research3(May 22, 1967):353–58. Outram, I.1970. Some effects of the fumigant sulphuryl fluoride on the gross metabolism of insect eggs. Fluoride3(2):85–91. Parker, T. A.1987. Integrated pest management for libraries. Paper presented at Preservation of Library Materials Conference, Austria, April 7–10, 1986. International Federation of Library Associations and Institutions publications (The Hague, Netherlands)41(2):103–23. Parker, T. A.1989. Private communication with T. A. Parker, entomologist, Pest Control Services, Inc.
Peltz, P., and M.Rossol. 1983. Safe pest control procedures for museum collections. New York: Center for Occupational Hazards. Pest Control. 1977. Dewey defends Hearst Castle against lyctus beetles. Pest Control45(6):30, 31, 48. Roth, H.1973. Fumigants for quarantine control of the adult brown dog tick: laboratory studies. Journal of Economic Entomology66(6):1283–85. Sanders, S.1987. Effects of CO2 fumigation on pH. ICOM Committee for Conservation, 8th Triennial Meeting, Sydney, 3:945–946. Scheffrahn, R., W.Osbrink, R.Hsu, and N.-Y.Su. 1987. Desorption of residual sulfuryl fluoride from structural and household commodities by headspace analysis using gas chromatography. Bulletin of Environmental Contamination and Toxicology39:769–75. Scheffrahn, R., R. C.Hsu, W.L.A.Osbrink, and N.-Y.Su. 1989. Fluoride and sulfate residues in foods fumigated with sulfuryl fluoride. Journal of Agricultural and Food Chemistry37(1):203–6. Scheuerman, E. H.1986. Suicide by exposure to sulfuryl fluoride. Journal of Forensic Sciences31(3):1154–58. Story, K.1985. Approaches to pest management in museums. Washington, D.C.: Conservation Analytical Laboratory, Smithsonian Institution. 85–101. Su, N.1990. Fumigant efficacy of sulfuryl fluoride against four beetle pests of museums (Coleoptera: Dermestidae, Anobiidae). Journal of Economic Entomology. in press. Torkelson, T. R., H. R.Hoyle, and V. K.Rowe. 1966. Toxicological hazards and properties of commonly used space, structural and certain fumigants. Pest Control34(7):13–18, 42–50. Valentin, N.1988. Unpublished results from tests using Vikane as a sterilant, Getty Conservation Institute. Valentin, N., and F.Preusser. 1989. Insect control by inert gases for museum archives and libraries. Unpublished manuscript.
Zycherman, L. and R.Schrock, eds.1988. A guide to pest control in museums. Washington, D.C.: Foundation of the American Institute for Conservation of Historic and Artistic Works and the Association of Systematics Collections. AUTHOR INFORMATIONMICHELE R. DERRICK, graduated from Oklahoma State University with a M.S. degree in Analytical Chemistry. She is currently an Associate Scientist at the Getty Conservation Institute. Her current research is in the development of new methods for the characterization and identification of organic materials in cultural objects primarily using infrared spectroscopy and pyrolysis gas chromatography. Address: Getty Conservation Institute, 4503 Glencoe Avenue, Marina del Rey, 90292. HELEN D. BURGESS graduated from the University of Lethbridge (Lethbridge, Alberta) with an honors B.A. degree in Chemistry. She went on to obtain a M.Sc. in protein chemistry from the chemistry department of the University of British Columbia (Vancouver, Canada) and a Masters of Art Conservation from Queen's University (Kingston, Ontario), specializing in conservation science. In 1978 she joined the staff of Conservation Processes Research, where she is currently employed as Senior Conservation Scientist. Address: Canadian Conservation Institute, Department of Communications, 1030 Innes Road, Ottawa, Ontario, Canada, K1A OC8. NANCY E. BINNIE graduated from Carleton University (Ottawa, Canada) with a honours B.Sc. degree in chemistry. She subsequently obtained an M.Sc. degree from Carleton specializing in Raman and fluorescence studies of Cholorophyll a monomers and aggregates. In 1987, she joined the staff of Conservation Processess Research, where she is currently working as Assistant Conservation Scientist. Address: Canadian Conservation Institute, Department of Communications, 1030 Innes Road, Ottawa, Ontario, Canada, K1A OC8. MARY T. BAKER received her B.S. degree in Chemistry in 1980 and her Ph.D. in 1986 in Materials Science with a specialty in Polymer Science from the Institute of Materials Science at the University of Connecticut. She has worked at CAL as a research chemist since 1987, collaborating with conservators on projects such as the effects of fumigation on materials; treatment and characterization of coated papers; light bleaching of paper; and methods development for analysis of microsamples of paints, varnishes, and other materials. Her current research is on the modern polymeric materials in air and space artifacts, their aging mechanisms, storage, treatment and display. Address: Conservation Analytical Laboratory, Smithsonian Institution, Washington, D.C. 20560.
 Section Index Section Index |