CONTROLLING THE REFRACTIVE INDEX OF EPOXY ADHESIVESJohn M. Messinger, & Peter T. Lansbury
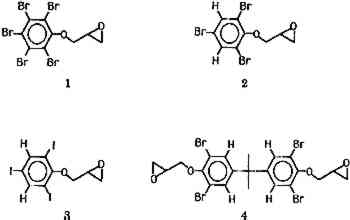
2 DISCUSSIONIT HAS LONG BEEN REALIZED that liquids and glassy solids that contain elements with high atomic numbers will have higher refractive indices. For example, if the hydrogens in a typical hydrocarbon are replaced by halogens, a material with a higher refractive index will be obtained. High density materials also tend to have higher refractive indices. Thus, polymers that are highly cross-linked or have longer chain lengths, and therefore higher densities, are observed to have higher refractive indices than their counterparts that are not as highly cross-linked or have shorter chain lengths.3 Epoxy adhesives were chosen for this initial study because two of the most popular adhesives for glass are epoxide based, and epoxides containing several halogens can be prepared easily. It was assumed that incorporation of an epoxide group into the additive molecule would cause the additive to have minimal effects on the tensile strength of the final adhesive. Four compounds were initially investigated as potential additives. The first three are halogenated derivatives of 1,2-epoxy-3-phenoxypropane: 1,2-epoxy-3-pentabromophenoxypropane (compound 1), 1,2-epoxy-3-[2,4,6-tribromophenoxy]propane (compound 2), and 1,2-epoxy-3-[2,4,6-triiodophenoxy]-propane (compound 3). Compound 4 is the diglycidyl ether of tetrabromo bisphenol A. The hydrogen analogues of these compounds are present in many commercially available epoxy adhesives.4
Precursors to these materials are particularly inexpensive due to their industrial use as fire retardants.5 All four were prepared from the corresponding phenol and epichlorohydrin in ca. 90% yield (scheme I), by an established procedure.6 |