PARTICLE CHARACTERISTICS OF PRUSSIAN BLUE IN AN HISTORICAL OIL PAINT
Frank S. Welsh
4 DISCUSSION
4.1 Appearance: 18th-century vs. 20th-century Prussian blue
THESE RESULTS raise the important question: Why does Prussian blue from the 18th-century samples differ so much in size and shape from that of the 20th century sample? Has a change in ingredients or preparation changed the aggregate appearance of this compound?
A quick glance at a mid 18th-century recipe for Prussian blue is helpful here. In his book, The Handmaid to the Arts, London, 1758, Robert Dossie describes the pigment's preparation, “as practiced on the continent and in this country.”
The first method employed to obtain Prussian blue was first to calcine dried bullock's blood with potash, in a crucible at red heat. The mixture was now poured into a quantity of water. During the heat, the animal substance afforded the prussic acid, which was caused by the potash forming the prussiat of potash. This compound dissolved in the water, constituted what was then called the Prussian lixivium. To this was added a mixture of sulphate of iron and sulphate of alumine, in a state of solution. The prussic acid takes the iron, while the potash combines with the sulfuric acid. The excess of alkali precipitates the alumine from the alum, which becomes mixed with the prussiat of iron, and forms the substance which when separated from the liquid, washed and dried, forms a blue concrete mass, called Prussian blue…
In contrast, note the preparation of Prussian blue in Gettens and Stout's Painting Materials:
[Prussian blue] is now commonly made by the action of an oxidizing agent, such as potassium bichromate and sulfuric acid upon a mixture of copperas (ferrous sulphate), sodium ferrocyanide, and ammonium sulphate, giving a blue with the approximate formula Fe(NH4)fe(CN)6. The pigment which is precipitated from dilute solutions of those salts is a deep blue, finely divided compound which, after it has settled and after the mother liquor is drawn off, is washed, filtered, and dried. The product is amorphous in colloidal aggregates and so finely divided that it has almost the characteristics of a dye. By control of conditions of precipitation and oxidation, variations in shades and physical characteristics of the blue may be had.…blue-black, but with some varieties, especially those prepared with bleaching powder as the oxidizing agent, have a reddish, coppery lustre. It is a transparent color but has very high tinting strength.
Clearly, the preparation methods are different, but are these differences dramatically sufficient to alter Prussian blue particles' size and shape? Dr. Robert L. Feller, Director of the Center on the Materials of the Artist and Conservator, Mellon Institute, Pittsburgh, agrees that the difference in aggregate shape between 18th-century vs. 20th-century Prussian blues may well lie in the old method's choice of material, namely blood (protein), and possibly in the mixing and accidental meeting of ions.17 Today, by contrast, precipitation methods are much more clean and direct. Dr. McCrone concurs.18 Dr. Feller has kindly sent a sample of Prussian blue made by Bernard Keisch, a former research fellow at the Mellon Institute, following the old method using bullock's blood (Fig. 6). The pigment agglomerates of this sample are also large and angular.
Fig. 5.
Pigments identified as charcoal black, Prussian blue, white lead, and calcium carbonate, from the resette, Tower Stair Hall, reproduced at the same magnification as Figure 2.
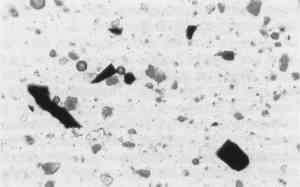 |
Fig. 6.
Prussian Blue made by Barnard Keisch, Mellon Institute, following an 18th century recipe, reproduced at the same magnification as Figure 2
 |
|

