PARTICLE CHARACTERISTICS OF PRUSSIAN BLUE IN AN HISTORICAL OIL PAINTFrank S. Welsh
ABSTRACT—This paper outlines current research to identify the pigments, specifically the blue pigment, used to make the original 18th-century finish paints in the Long Gallery and in the Tower Stair Hall at Independence Hall in Philadelphia. The restoration of these rooms' blue and blue-green painted finishes was carried out from the mid-1950s to the early 1970s under the guidance of the National Park Service Historical Architect, Penelope Hartshorne Batcheler. The recent analyses confirmed that a blue pigment in these paints was one very commonly used in the 18th-century, Prussian blue. This study and confirmation not only establishes one of the very earliest uses of the pigment in this country in that century, but it brings to light the obvious differences in physical appearance between 18th-century Prussian blue agglomerates (large and angular)1 and the small, amorphous 20th-century Prussian blue agglomerates. The research also establishes light microscopy as the method of choice for analyzing architectural paint pigments. 1 INTRODUCTIONTHE ANALYSIS OF HISTORIC ARCHITECTURAL PAINTS involves not only the identification of layers and colors of paint, but also it requires the accurate analysis and identification of the component pigments in the paint films. This is either out of historical, empirical interest or else out of an interest to more accurately understand a paint film's original color. Most often, though, the identification of pigments is undertaken to help prove the age of the paint film, since many pigments are commercially prepared and were not, therefore, necessarily available before a certain time. Thirty years ago, an attempt was made to identify an original paint's component pigments at Independence Hall; however, the findings from the analyses were inconclusive. The equipment and information available for the analysis at that time was not much different than that used for this recent study, which was undertaken in an attempt to answer the lingering questions. What has changed in the intervening years is our increased knowledge of the materials to make architectural paints 200 years ago and, consequently, what we might expect to find in them. With our enhanced knowledge and expectations, the full capabilities of the variety of scientific instruments available to us can be best put to use. 2 LONG GALLERYTHE LONG GALLERY (Fig. 1), a second-floor room running the width of Independence Hall, was Colonial Pennsylvania's version of Versailles Palace's Hall of Mirrors. The site of 18th-century banquets and concerts, the room also served on a day-to-day basis as a reception and waiting room. The office of Pennsylvania's governor adjoined it—Independence Hall was Pennsylvania's State House—and legislators probably
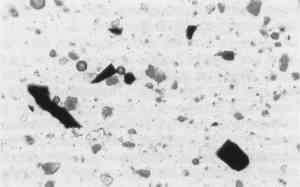
During the 1950s and 1960s, the National Park Service (NPS) restoration architects found on the window sash what appeared to be the room's original blue color remaining in the grain of wood. Subsequent microscopical paint analysis on other pieces of original trim established that this medium blue-tinted oil paint was the first finish on all wood. (There are three exceptions: The baseboards and the top surfaces of the chair rails and window sills were all originally dark brown.) In the opinion of all who looked at it, the blue pigment used in this original trim color was Prussian blue. However, it was never microchemically analyzed until 1985, when Mrs. Penelope Batcheler, NPS Historical Architect, pursued further analysis of the pigment with the author. Stereo microscopical examination of a small piece of a 1730s window splayed jamb board from the Long Gallery (cat. #8428-A) confirmed Mrs. Batcheler's original finding that the first finish is ca. 1730s medium blue oil paint applied over a thin red iron oxide prime coat. Within this blue finish paint, there were large blue-black pigment agglomerates averaging 250 micrometers in diameter. Several of them were removed with a tungsten needle for further examination. One sample was mounted on a glass slide in a permanent mounting medium (Aroclor� 5442), and inspected A comparison of the unknown sample was made to known samples of natural ultramarine blue and Prussian blue. The unknown blue pigment has optical characteristics similar to both. Standard microchemical tests on the blue pigment agglomerates were carried out.1 These established that the unknown blue pigment was Prussian blue rather than natural ultramarine. Also known as Berlin blue, Paris blue, Antwerp blue, and Chinese blue, Prussian blue is the earliest modern synthetic color.2 It is a complex chemical compound, ferric ferrocyanide (Fe4(Fe[CN]6)3), first mentioned in 1710, but its preparation was kept secret until 1724. A London manufacturer named Wilkenson began production, and gradually more and more color firms followed suit. By 1750, Prussian blue must To verify the identification of Prussian blue in the Long Gallery paint, Dr. Walter C. McCrone at the McCrone Research Institute in Chicago was consulted. The paint sampled was the same first (original) 1730s finish above a red oxide primer. Stereo microscopical inspection at 70x again revealed within the film large blackish-blue pigment agglomerates. These were removed with a tungsten needle along with some of the surrounding blue paint for examination by polarized light microscopy, microchemical testing, and electron microprobe analysis. Mounted on a glass slide in Aroclor� 5442, the first Long Gallery sample revealed two pigments, a pseudo-opaque white and a blue (Fig. 2).5 The sizes of the individual anisotropic white particles generally ranged from 0.9–3.8 micrometers. Their optical properties, e.g. refractive index of ≅2.00, showed they were white lead, 2PbCo3-Pb(OH)2.6 The whole paint film itself contains close to 75%–85% of this pigment.
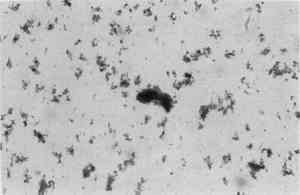
The blues were large isotropic glassy flakes with well-defined edges and corners and a refractive index of 1.56–1.57 as determined by dispersion staining.7 Their purple-blue color was slightly mottled, not consistently uniform. Their size generally varied from 4.0 micrometers all the way up to an incredibly large agglomerate of 46.8 micrometers. Comparison was made of the above blue particles with those of other blue pigment samples, natural ultramarine (ground lapis lazuli)8 and modern Prussian blue.9 In color and shape the Long Gallery blue pigment sample was more like the ultramarine. Like the sample, ultramarine particles are glassy, have well-defined edges and corners, and are isotropic. There, however, the similarity ended. While the Long Gallery blue color was mottled, natural ultramarine's is consistently uniform. Their respective refractive indices also are different, that of ultramarine being 1.50.10 In a comparison of the shape of the Long Gallery 18th-century blue pigment sample to the shape of the 20th century Prussian blue sample (Fig. 3),11 a distinct difference is apparent. The 18th-century pigment has sharp, well defined edges, and the 20th-century Prussian blue has rounded edges—almost amorphous in agglomerate shape. The color of the 18th-century blue is mottled, but the 20th-century Prussian blue is very dark and more uniformly blue. Both have an identical refractive index of 1.56.
When viewed at 400x magnification, the shape of the Long Gallery 18th-century blue pigment resembled natural ultramarine but could be distinguished from ultramarine by the unknown pigment's color and refractive index which strongly suggested Prussian blue. This was confirmed by standard microchemical tests.12 Electron microprobe analysis verified this identification of the blue and of the white as white lead.13 3 TOWER STAIR HALLFEW, IF ANY, have postulated that Prussian blue was also the pigment used in the original finish paint found in Independence Hall's grand stair area, the Tower Stair
Over the past three decades, a number of laboratories have analyzed the Stair Hall's pigments. Samples of the original paints were submitted by Mrs. Batcheler to the National Lead Company Research Laboratory (December, 1956), to the Freer Gallery, whose laboratory was then headed by Rutherford P. Gettens (November, 1957), and to The Glidden Paint Company of Reading, Pennsylvania (May, 1958). The conclusions of all analyses were contradictory and never resolved. National Lead concluded the blue pigment in question was natural ultramarine. Glidden Labs concurred, even after conducting X-ray diffraction and spectrographic analyses. Dr. Gettens disagreed. He found no blue in his microscopical analysis-only iron oxides and charcoal black. 14 The conflicting analysis left the identity of the Stair Hall's original paint's pigments a mystery. In 1985, the files were reopened to attempt to find an answer. Stereo microscopical inspection indicated the first finish to be a light grayish-greenish blue oil paint over a very light oil primer. Through the stereoscope we saw large black pigment agglomerates. Agglomerates of the pigments in the paint were too small to see easily at 20–80x. The absence of large blue pigment agglomerates, similar to those found in the 1730's Long Gallery paint, suggests this Tower Stair Hall paint layer, potentially from the 1750s, may have been made with a different blue pigment or else the same one but more finely ground before dispersal into the liquid paint.15 A tiny sample (0.5mm) of the entire paint layer was removed with a tungsten needle and mounted on a glass slide in Aroclor� for inspection with the polarized light microscope. Easily seen were white, black, The characteristics of the black pigment were those of charcoal black. The pieces were large and very angular, very unlike lamp black, which is very tiny and rounded.16 The yellow and red pigments looked like yellow and red iron earths. The white had characteristics of white lead. The blue had identical optical and shape characteristic of the blue sample from the Long Gallery's window jamb board, with one exception. This sample's agglomerates size was generally much smaller, ranging from 3–11 micrometers with 75% closer to 4 micrometers—a factor contributing to the potential for its misidentification. These particles were examined one by one with the polarized light microscope and with microchemical testing and found to be the following: black, charcoal black; red, red iron earth and haematite; yellow, yellow ocher; white, white lead, and the blue, Prussian blue. 4 DISCUSSION4.1 Appearance: 18th-century vs. 20th-century Prussian blueTHESE RESULTS raise the important question: Why does Prussian blue from the 18th-century samples differ so much in size and shape from that of the 20th century sample? Has a change in ingredients or preparation changed the aggregate appearance of this compound? A quick glance at a mid 18th-century recipe for Prussian blue is helpful here. In his book, The Handmaid to the Arts, London, 1758, Robert Dossie describes the pigment's preparation, “as practiced on the continent and in this country.”
In contrast, note the preparation of Prussian blue in Gettens and Stout's Painting Materials:
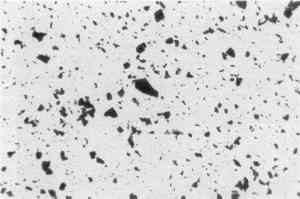
Clearly, the preparation methods are different, but are these differences dramatically sufficient to alter Prussian blue particles' size and shape? Dr. Robert L. Feller, Director of the Center on the Materials of the Artist and Conservator, Mellon Institute, Pittsburgh, agrees that the difference in aggregate shape between 18th-century vs. 20th-century Prussian blues may well lie in the old method's choice of material, namely blood (protein), and possibly in the mixing and accidental meeting of ions.17 Today, by contrast, precipitation methods are much more clean and direct. Dr. McCrone concurs.18 Dr. Feller has kindly sent a sample of Prussian blue made by Bernard Keisch, a former research fellow at the Mellon Institute, following the old method using bullock's blood (Fig. 6). The pigment agglomerates of this sample are also large and angular.
5 CONCLUSIONCARE MUST BE USED in identifying blue pigments in early paint films. In particular, the present paper has shown that 18th-century Prussian blue particle agglomerates, which can be very large or small and angular, may be mistaken for natural ultramarine which they more closely resemble morphologically than they do particles of modern Prussian blue, which is very amorphous in shape. Careful use of analytical techniques, especially polarized light microscopy, can help prevent misidentifications. Blue paint films containing Prussian blue in the form of larger angular particle agglomerates may have been prepared from the pigment made by the earliest described process for its preparation. Such paint films therefore, may be relatively early. ACKNOWLEDGEMENTSTHE AUTHOR wishes to thank and acknowledge the many hours of expert assistance of Dr. Walter C. McCrone and Mrs. Penelope H. Batcheler, and for the critical insight of Dr. Robert L. Feller and others, for without their support, this project could not have been completed. NOTES. 1 Joyce Plesters, “Cross-sections and Chemical Analysis of Paint Samples,” in Studies in Conservation 2, No.3, April, 1956, pp. 110–157, Microchemical tests used included hydrochloric acid which bleaches ultramarine blue, and sodium hydroxide which discolors Prussian blue to a dark orange yellow. NOTES. 2 Rutherford J. Gettens and George L. Stout, Painting materials, A Short Encyclopedia (New York, 1966) pp. 149–151. NOTES. 3 Gettens and Stout, p. 151. NOTES. 4 Penelope H. Batcheler, Historic Structure Report, Old North Church, (National park Service, Denver Service Center, 1981) pp. 193–194. NOTES. 5 Walter C. McCrone, Lucy B. McCrone, and John Gustav Delly, Polarized light Microscopy (Ann Arbor: Ann Arbor Science Publishers Inc., 1979). p. 35. A pseudo-opaque white appears to be opaque because the light that is transmitted though the thicker agglomerated particles is bent and does not enter the objective lens. NOTES. 6 Gettens and Stout, p. 147. NOTES. 7 McCrone, McCrone and Delly, p. 169. The refractive index determination using dispersion staining was carried out by Dr. Walter C. McCone in the conservation lab at N.Y.U. NOTES. 8 Natural Ultramarine, Forbes collection, 7.01.13. NOTES. 9 Prussian Blue, Forbes Collection, Azzuri de Prerlino and Bibbiena. NOTES. 10 Gettens and Stout, p. 148b. NOTES. 11 Prussian blue, Slide reference set of 1982 from The McCrone Research Institute. NOTES. 12 Plesters. HC1 and NaOH microchemical tests, p. 137. NOTES. 13 Electron microprobe analysis preformed by Walter C. McCrone and McCrone Associates. 1984. NOTES. 14 Penelope Hartshorne (Batcheler). Log of Restoration, Independence Hall, Tower Stair Hall, 1956–57. NOTES. 15 The size of the particle agglomerates visible in the paint film do not necessarly indicate an early or late date of manufacture. I have seen very large Prussian blue agglomerates in early 19th century blue paint films from buildings in Tennessee and in Texas. NOTES. 16 Charcoal Black (Forbes: 2.01.7) and Lamp Black, McCrone Research Institute. NOTES. 17 Private communication with Dr. Robert Feller. 1986. NOTES. 18 Walter C. McCrone, written correspondence to the author: “Even with the same process, there can be differences in the product, especially particle size. This would be due to variations in temperature and time.” 1987.
 Section Index Section Index |