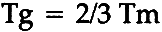GENERAL EFFECTS OF AGEING ON TEXTILESRandall R. Bresee
2 TYPES OF AGEING2.1 Physical AgeingThe chronological age of a material is considered to be the time elapsed since the material was formed. The physical age, on the other hand, is viewed as the time elapsed since the glass transition temperature of the material, Tg, was last exceeded. The glass transition occurs in a fairly narrow temperature range and involves substantial physical differences in polymer properties above and below the transition temperature. At temperatures below the Tg, the noncrystalline segments of polymers are brittle like glass, whereas they are rubbery at temperatures above the Tg. The cause for these differences is polymer segmental mobility. These properties result because at temperatures below the Tg, mobility in relatively long segments of polymer chains does not exist whereas above the Tg, mobility occurs freely. the glass transition is strictly a physical phenomenon and applies to all polymeric materials without regard to the source of a polymer. That is, fibers of both natural and man-made origin exhibit the glass transition. All polymeric objects containing at least some noncrystalline material existing at a temperature below the object's Tg have undergone some physical ageing. The last crystalline materials exhibit the greatest changes in properties during physical ageing. Physical ageing occurs universally in the glassy state irrespective of the chemical nature of a polymer. Physical ageing has been seen in a large variety of materials, including fibers, sugar, and shellac.1 Since the glass transition temperatures of most fiber-forming polymers are well above room temperature and all fibers contain some noncrystalline areas, most fibers would be expected to exhibit the effects of physical ageing from storage at room temperature. These effects result from the ordering of noncrystalline polymer chains and include decreases in free volume, enthalpy and molecular mobility.1, 2 A fiber that has physically aged can be expected to be harder, more dense, more stiff and to exhibit increasing viscoelastic relaxation times compared to the same fiber that has not been aged physically. One of the most interesting aspects of physical ageing is that it can be “erased” simply by heating a material to a temperature that exceeds its Tg. This may seem to be a rather harsh thermal treatment for many of the natural fibers since they typically have relatively high glass transition temperatures. For example, the Tg of wool is reported to be around 160�C3 and that of cotton around 230�C.4 The Tg of polymers can be depressed by the action of plasticizers, however, and most natural fibers absorb large quantities of water which acts as an effective plasticizer. It has been demonstrated experimentally,3 or can be calculated theoretically5 that the Tg's of most natural fibers are depressed to a temperature around room temperature by the absorption of water during wetting. At least some of the improvement in properties resulting from wet cleaning many aged textiles certainly must result from erasure of physical ageing and the consequent decrease in hardness, density and stiffness. Although the conservator must consider many other factors, the erasure of physical ageing constitutes a strong argument favoring wet cleaning historic textiles. While the magnitude of the benefit achieved from this practice will vary depending on the age of the textile (more benefit to more aged textiles), all physically aged textiles will become softer, less dense and more pliable after erasure of physical ageing. All textiles stored at temperatures below their glass transition temperatures (likely for nearly all natural fibers) will redevelop symptoms of physical ageing during storage, but this ageing may again be erased at a later date. Textiles may be kept in environments that do not allow physical ageing to occur. This would include any environment where the textile exists at a temperature above its Tg. This condition is achieved for most natural fibers around room temperature if they are wet. Many textiles have been recovered in remarkably good condition from wet environments. Although it has not been experimentally demonstrated, one would expect physical ageing of these textiles to be absent and this absence to have contributed to their good condition. Of course, the elimination of air from wet environments would be expected to contribute to the longevity of textiles since chemical ageing is thus also reduced. Another interesting aspect of physical ageing is that it proceeds predictably and measurably in samples during ageing times as short as a few minutes or as long as a few million years.1 In one study, microscopic measurements of tensile creep (elongation under a constant load) were investigated as a means of determining the physical age of short lengths of single fibers.6 In favorable circumstances, a textile's physical age may be approximately equated to its chronological age (i.e. Tg has not been exceeded since the fiber was formed), so the technique described may be used to provide an estimate of the chronological age of textiles of unknown origin. 2.2 Photochemical DegradationSince nearly every textile spends at least part of its life exposed to light, contributions of photochemical reactions to textile ageing would be expected to be common and to add significantly to textile deterioration in some cases. The fundamental source of deterioration of chemical or physical properties are chemical changes in polymer composition. In simple terms, chemical changes are those involving destruction and formation of covalent bonds. Unlike physical ageing, which occurs only in noncrystalline areas of polymer materials, photochemical degradation occurs in both crystalline and noncrystalline areas since electromagnetic radiation can penetrate both areas. One would expect The elucidation of specific reaction pathways in photochemical degradation has proven to be a difficult task.8 Consider, for example, the problem of wool fiber yellowing that results from exposure to light. Numerous researchers have attempted to determine the specific reactions responsible for this phenomenon, but none have been completely successful. However, one can discuss the general effects of photodegradation on fibers and obtain a basic idea of what to expect in terms of general structure and properties. Polymer molecular weight changes commonly result from photochemical degradation and substantially change the properties of fibers. The scission of bonds results in a decreased average molecular weight. If a fiber suffers a net decrease in molecular weight, one can expect major decreases in tensile strength and elasticity and a moderate decrease in elongation-to-break. The deterioration of these properties makes handling textiles without fiber failure difficult since the material becomes easily deformed and breaks under low stress. In addition to deterioration of these mechanical properties, chain scission results in increased chemical reactivity since new chemical bonds are formed and the degraded material is chemically more diverse. This can be seen in increased solubility, sensitivity to chemical reactions such as bleaching, and enhanced sensitivity to subsequent photochemical degradation. Whereas chain scission results in a decrease in polymer molecular weight, crosslinking results in a major increase in molecular weight. In crosslinking, previously unconnected segments of polymer are connected by formation of new covalent bonds between them. At low crosslinking levels, increases in strength, elasticity, and toughness are observed, whereas high crosslinking levels result in further increases in strength and elasticity but also embrittlement of the sample since the elongation-to-break decreases greatly. In addition, crosslinking results in a general decrease in chemical reactivity, which can be seen in decreased solubility and susceptability to further reactions. A good example of the influence of crosslinking of fiber properties can be obtained by comparing regular cotton with cotton that is crosslinked by a durable press treatment. In this case, the crosslinked cotton is insoluble and brittle compared to the regular cotton fibers. Since chain scission and crosslinking result in major changes in fiber properties and affect fibers in substantially different ways, research has been conducted to define conditions under which either chain scission or crosslinking is preferred over the other. Studies have shown that chain scission predominates over crosslinking in oxygen-rich environments whereas crosslinking predominates in oxygen-starved environments.9, 10 With regard to fiber morphology, one study showed that crosslinking efficiency is greater in the crystalline than in the noncrystalline sections of a polymer material.11 This apparently results because polymer segments are closer together in crystallites since the chain packing density is greater. In addition, studies have revealed that scission of noncrystalline polymer chains may result in stress cracking.12 These studies indicate that light affects fiber properties in many deleterious ways and thus should be avoided where possible. Covalent bonds are composed of electrons being shared between at least two atoms, and chemical changes resulting from photochemical degradation involve the two basic steps of absorption of energy by the electrons followed by bond breakage and new bond formation. General principles concerning absorption of electromagnetic energy (photons) by electrons apply. First of all, electrons can only absorb photons of relatively few and very specific energy values. Second, one can approximately predict absorption for any molecule of interest using some simple rules of thumb: single bonds in organic molecules have little or no absorption in the visible and atmospheric ultraviolet regions of the electromagnetic spectrum; isolated double bonds in orgranic molcules have no absorption in the visible region but may absorb in the ultraviolet region; conjugated double bonds in organic molecules may absorb strongly in the visible or ultraviolet regions of the spectrum; and inorganic compounds may absorb strongly in the visible or ultraviolet regions of the spectrum. Consideration of these rules of thumb leads one to expect little photochemical degradation of organic polymers which are entirely singly bonded. Examples include polyethylene and polypropylene. Materials made of these polymers are known to suffer severely at times from photochemical degradation, however. This has been blamed on the presence of inorganic compounds such as polymerization catalysts, terminators, and pigments which are incorporated into the polymer material during polymerization. The application of this point to historic textile conservation is that the presence of soil on textiles probably will result in the textiles being more sensitive to photochemical degradation than they would be if the soil was removed. This is an argument favoring the cleaning of historic textiles. In addition, it is an argument in favor of multiple rinsing during wetcleaning, using a good quality water that contains little or no impurities. Consideration of the general rules concerning absorption of light also leads one to expect possible photochemical degradation by ultraviolet light of any fiber containing isolated double bonds. The poor light resistance of wool, silk, nylon and polyester illustrate this problem, as all of these polymers contain double bonds in the form of the carbonyl moiety. The use of ultraviolet filters over light sources in buildings housing textile collections has been shown to decrease the damage caused by photochemical degradation. Finally, one would expect molecules containing conjugated double bonds, such as most dyes, optical brighteners and some polymers to absorb visible light. These materials would be expected to be very unstable in the presence of light. An object that has been somewhat degraded photochemically may be expected to be more sensitive to further light damage than the same object if not previously degraded. This arises because bonds are broken and new bonds are reformed during photochemical degradation, and the degraded object thus is chemically more diverse than the undegraded object. Since bonds abosrb relatively few and very specific wavelengths of light, an object can absorb a greater number of wavelengths of electromagnetic radiation when degraded than if not degraded: a greater number of bond types are present in a more chemically diverse object. In other words, the object becomes more sensitive to further photochemical degradation as it degrades. In general, this would justify requiring greater protection from both ultraviolet and visible light for historic artifacts than for similar new objects. Once photochemical energy is absorbed by some chemical bond in an object, the bond may break. Although it has not generally been shown how many bonds must be broken in typical fibers to produce an observable amount of deterioration of the fiber properties, a surprisingly small number of polymer skeletal bonds need to be broken to produce a decrease in the strength of some materials. In one study, only 4% of the amide linkages in Kevlar 49 fibers had to be broken to produce zero strength.13 Bonds that do not absorb electromagnetic radiation themselves may become involved in chemical reactions and be broken. This occurs because once a particular bond is broken, the radical fragments are extremely reactive and react with many other chemical species. If molecular oxygen is present, it is a convenient species for reaction, and many reactions collectively called photooxidation occur. The photooxidation of many polymers has been studied in detail and has been shown to be quite complex.14 One would expect textiles housed in oxygen-starved environments to have undergone little photooxidation. These environments include air-tight tombs, underground burials in impermeable clay soils, and cold water. Of course, some of these environments also completely exclude electromagnetic radiation so that photochemical ageing is completely eliminated while stored in the dark environment. Many textiles have been recovered in remarkably good condition from environments where photochemical ageing has been eliminated. 2.3 Thermal DegradationOne may classify thermal effects into two general classes. One class involves purely physical structural changes in the fiber substrate whereas the other involves chemical reactions. Since heat may penetrate throughout, structural changes would be expected to occur in both crystalline and noncrystalline areas of fibers. One must examine the major thermal transitions of polymer materials to understand changes in fibers that are purely physical. Although there are many transitions that can be identified in most polymer materials, the two most important ones are the glass transition, which pertains to noncrystalline segments of polymer, and melting (or the reverse process, crystallization, which pertains to crystalline polymer. The phenomenon of the glass transition has great consequences for textile conservators because it occurs at a low enough temperature for most fibers that it can be exceeded under fairly common conditions. One important consequence is that textiles that are too brittle to handle uner normal conditions may be handled easier by temporarily changing them from the glassy state to the rubbery state so they are less brittle. This is done by subjecting them to conditions where they exist above their Tg. As mentioned previously, this may be accomplished by heating them to a temperature slightly above their Tg or by plasticizing them with water, for example, so that their Tg is depressed to a lower temperature near room temperature. Another important consequence of the glass transition is that the increased segmental mobility exhibited by polymer materials at temperatures above the Tg allows much greater diffusion of molecules through the polymer matrix than when at temperatures below the glass transition. In other words, molecules such as dyes or finishes may diffuse out of fibers more easily, or other molecles such as bleaches, oils, soils, or detergents may diffuse into fibers more easily. This, of course, is the source of dye bleeding in some textiles during wet cleaning. Similarly, one must guard against the diffusion of harmful substances out of other polymeric materials when they are above their Tg. For example, it was recently determined that an antioxidant was released from polyethylene film (Tg = −85 C) which was used to store garments at room temperature, and the antioxidant caused yellowing of the garments.16 Similar problems may result from adhesives, reinforcements and other polymer materials used in conjunction with historic textiles. A third consequence of the glass transition that is of concern to textile conservators The other important polymer thermal transition is melting (its reverse process is crystallization). These are phenomena that pertain to crystalline parts of fibers and involve the physical transformation between crystalline polymer segments and noncrystalline segments with consequent changes in stiffness and hardness. Although these processes occur at higher temperatures than the glass transition, they are nevertheless encountered sometimes when handling textiles. For example, the glazing of polyester/cotton fabric during ironing at too high a temperature involves the melting of crystallites in some of the polyester fibers. Although melting usually occurs through a quite broad temperature range, a melting point is defined for most polymers.4 The melting point always occurs at a temperature well above the glass transition temperature, the usual relationship being17
For many fibers, such as the protein and cellulose fibers, the melting point exists at a temperature above which the polymer undergoes thermal decomposition involving chemical changes. Consequently, although a melting point exists for these polymers, they chemically decompose at temperatures below their Tm's. While the example involving glazing polyester fibers is rather severe, note that melting actually may begin at lower temperatures. The lower temperature limit is the temperature at which segmental mobility is achieved, Tg, since segmental mobility is required for melting. In other words, whenever a polymer material is heated to a temperature above its Tg, small, imperfect crystallites potentially may melt and this occasionally may present problems in the treatment of textiles. One effect of melting oriented materials is that it usually is accompanied by polymer disorientation and decreasing orientation results in decreases in strength as well as changes in many other mechanical properties. The same temperature limit applying to the glass transition applies to crystallization; that is, the Tg is the lower limit of crystallization. Consequently, one must watch for melting or crystallization of any crystallizable polymer material when it is at a temperature that exceeds the Tg. Of course, this applies to other materials besides fibers. It should be noted that many compromises usually must be made in regard to structural considerations during conservation treatments. For example, although it has not been investigated experimentally, one might anticipate problems during physical age erasure of fibers whose crystalline structure is severely degraded, since improvements of mechanical properties resulting from physical age erasure could be offset by deterioration of mechanical properties resulting from melting and polymer disorientation. 2.4 Chemical AttackSome fibers are relatively stable to chemical attack. For example, some high performance fibers actually are spun from concentrated sulfuric acid. This certainly indicates exceptional chemical stability. Other fibers, however, are susceptible to attack by a multitude of chemical species. Reactivity generally increases as the chemical diversity of molecules increases. On this basis, cellulose fibers are less susceptible to chemical attack than protein fibers and polyethylene fibers are even less reactive. In addition, susceptibility to chemical attack increases as degradation in a fiber increases since the chemical diversity of a material more than likely increases as degradation proceeds. Another general rule is that susceptibility to chemical attack increases with decreasing crystallinity. This occurs since chemical species attacking a material can not directly penetrate the dense structure of crystallites and can penetrate the less dense noncrystalline areas considerably easier. Given enough time, however, crystallites can be destroyed by attack at the crystal surfaces with the destruction then slowly proceeding inward as each crystalline layer is destroyed consecutively. Flax is more resistant to chemical attack than viscose rayon since the latter is significantly less crystalline than the former, even though both are cellulosic. A final rule governing chemical attack is that the rate of chemical reactions generally increase with increasing temperature. Consequently, chemical degradation can be decreased by minimizing temperatures when textiles are exposed to reactive chemical species. 2.5 Mechanical StressIt is instructive to discuss this topic in terms of the viscoelastic nature of polymers. That is, polymer materials respond to stress on two different time scales. Responses that essentially are instantaneous are called elastic whereas responses that are delayed in time are called viscous. The most fundamental point to keep in mind with regard to the viscous nature of fibers is that their response is time dependent. In other words, the mechanical response to a stress depends upon the rate of stress. This phenomenon has numerous implications for the textile conservator. For example, consider a historic textile that is folded and stored in that condition for a long time. Folding causes stress to be placed upon fibers near each fold, and during storage the fibers respond to this stress by straining over time until complete stress relaxation has occurred (stress reaches zero). If a stress equal and opposite to the initial stress plced on a fold is applied to the textile in an attempt to unfold it, it will require the same amount of time for relaxation of the new stress (removal of the fold) as was required for complete relaxation of the original stress. Increasing the magnitude of the second stress, of course, will decrease the time required for fold removal, but reversibility of the procedure is not as complete in mechanical terms since polymer chain scission, changes in gross size and shape, or fiber fracture may occur. One way to decrease the time required for fold removal is to increase the mobility of the polymer molecules so the rate of stress relaxation associated with fold removal is increased. A convenient means of doing this is to heat the polymer or to plasticize it, especially if the glass transition is reached so that a major increase in polymer segmental mobility is obtained. The most familiar example of this involves applying steam (heat and plasticizer) to modern wool garments to remove wrinkles. Similarly, wet cleaning involves plasticization and depression of the Tg so mechanical relaxations occur much faster than if the same stress is applied to the dry textile. Considering the influence of soil on the mechanical behavior of textiles is important for conservators. At least some soil adheres to more than one fiber and thus decreases the mobility of the fibers with respect to one another. If a textile is stressed, such as when folded or unfolded, the flexibility of the fabric is decreased compared to an unsoiled textile since the fibers can not reorder as much to reduce the local stress by |