VAPOR PHASE CONSOLIDATION OF BOOKS WITH THE PARYLENE POLYMERSBruce J. Humphrey
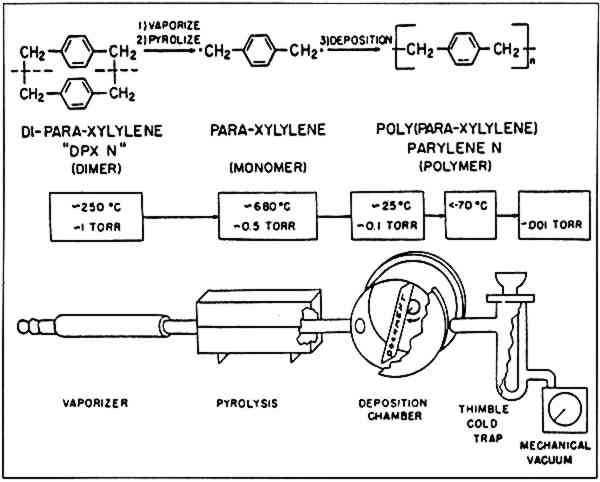
3 A BRIEF EXPLANATION OF THE DEPOSITION PROCESSTHE FOLLOWING IS A BRIEF DISCUSSION of the mechanics and chemistry of the deposition process. This mechanism remains the same regardless of the nature of the material being treated. The parylene polymers are deposited directly from a gas phase. No liquid phase has ever been isolated and no solvents are involved. Polymerization must take place in a “medium vacuum” (4–10 microns mercury). Materials to be coated are placed in the chamber at the right of the illustration (Fig. 1). Parylene dimer is placed in the vaporizer zone at the left. The system is closed and evacuated till the proper vacuum is reached. The temperature of the vaporization zone is gradually raised to around 160�C. The parylene dimer then begins to sublime forming a gas. As the pressure increases in this zone, the gas moves downstream into
|