VAPOR PHASE CONSOLIDATION OF BOOKS WITH THE PARYLENE POLYMERSBruce J. Humphrey
ABSTRACT—In a previously published paper on the application of parylene technology to conservation1, the author proposed that parylene vapor polymerization might have applications in the consolidation of bound books which have become weakened or embrittled due to the high acid content of the paper. When applied to paper substrates, this process is irreversible. It is not known at this time whether the benefits derived from use of the material will be great enough to overcome the reluctance to use such an irreversible procedure.The princpal purpose of the present paper is to introduce to the conservation community the basic concept that weakened or brittle books may benefit by gas phase treatment with the paraxylylenes. Areas under discussion are: explanation of parylene, characteristics of the polymer, the deposition process, response of books to the application of a medium vacuum, technical problems posed by bound volumes, the gaseous diffusion phenomenon, the problem of polymer thinning, determination of dimer charge, immersion tests to determine coating efficiency, moisture content and deposition efficiency with bound volumes, tensile strength and fold endurance, and general conclusions drawn from this initial investigation into the gas phase polymerization of parylene polymers within bound books. 1 INTRODUCTION AND BACKGROUND INFORMATIONPARYLENE IS THE GENERIC NAME for the various members of a Polymer series developed by Union Carbide Corporation. (PARAXYLYLENES). Parylene has been in commercial use for about 20 years. The primary application for this polymer has been in the electronics industry where the process is becoming increasingly important as a means of protecting delicate microcircuitry from all forms of hostile environments. To the best of the author's knowledge, parylene has not until recently been considered as a possible conservation material. The author's experience with parylene and its properties led to the investigation of potential applications for this polymer in the various conservation fields. Brittle books (books whose paper fractures after one or two folds) seem to be likely candidates. The fact that this polymer is deposited from the gaseous phase indicated that it might be possible to treat entire books without disturbing the binding. It is the author's understanding that the acid content in paper eventually results in the cellulose fibers breaking into smaller and smaller units, thus causing the paper to become brittle. Consolidative treatments seek to reunite these fibers in order to regain some of the lost strength. It is the author's belief that the parylene family of polymer holds great promise for accomplishing this consolidation in bound books. This paper is a brief account of the first tentative steps toward this goal. As stated earlier the process, on paper, is an irreversible polymerization reaction, so a great deal more will have to be learned prior to actual conservation use. Exhaustive studies of parylene are now in progess at the J. Paul Getty Conservation Institute with an emphasis on qualification for ethnographic purposes. The Library of Congress is investigating the process described in this paper as a potential means of “mass consolidation” of that portion of their collection which has become brittle. It is hoped that the results described here will stimulate further interest and research into the application of this polymer for conservation, and that readers will be informed about a new technology that may have some future impact on their various fields of endeavor. 2 SOME GENERAL CHARACTERISTICS OF THE POLYMER PHASE OF PARYLENE
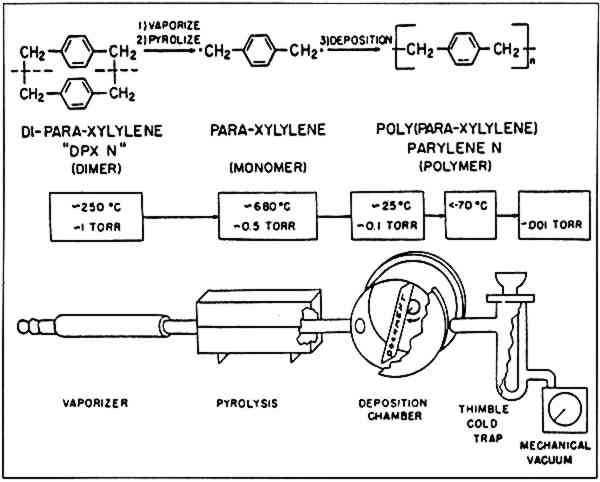
3 A BRIEF EXPLANATION OF THE DEPOSITION PROCESSTHE FOLLOWING IS A BRIEF DISCUSSION of the mechanics and chemistry of the deposition process. This mechanism remains the same regardless of the nature of the material being treated. The parylene polymers are deposited directly from a gas phase. No liquid phase has ever been isolated and no solvents are involved. Polymerization must take place in a “medium vacuum” (4–10 microns mercury). Materials to be coated are placed in the chamber at the right of the illustration (Fig. 1). Parylene dimer is placed in the vaporizer zone at the left. The system is closed and evacuated till the proper vacuum is reached. The temperature of the vaporization zone is gradually raised to around 160�C. The parylene dimer then begins to sublime forming a gas. As the pressure increases in this zone, the gas moves downstream into
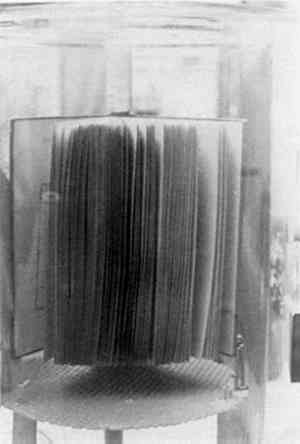
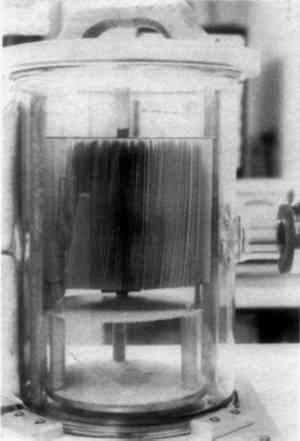
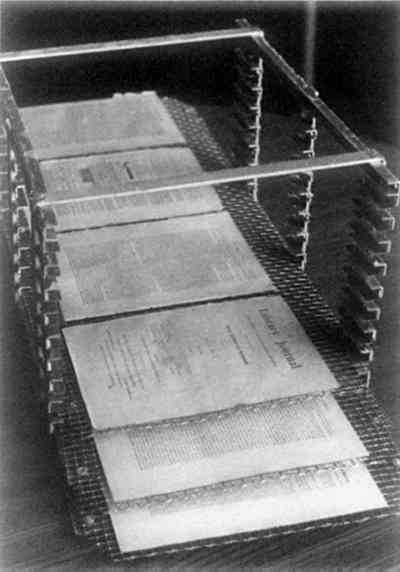
4 RESPONSE OF A BOUND VOLUME TO VACUUMTHE FIRST STEP in this investigation involved placing a book in the vacuum chamber to determine what effect this would have and to determine if it would be possible to arrange the individual pages to allow adequate penetration of the parylene molecules. Some “interleaf spacing” would have to be maintained as parylene will not penetrate a closed book. For this test, the book was placed on a platform in an upright position and opened about 180� so that the binding supported the book and the leaves were free to move within the arc created by the book covers (Fig. 2). An attempt was then made to arrange the leaves more or less equidistant from one another. This became a frustrating task and it was decided to simply place the mounted book in the chamber and evacuate the air to see what immediate effects the vacuum would have on the book. This was done, and it was immediately discovered that the leaves of a book will automatically arrange themselves in a fanlike fashion as the air is evacuated (Fig. 3). The cause of this phenomenon is thought to be outgassing of air and moisture as the chamber pressure drops. The “fanning” phenomenon occurs generally in the first 30–45 seconds of the evacuation cycle. It was this unusual and unexpected phenomenon that made it possible to attempt the “in situ” polymerization of parylene in books. A book when closed or with “bunched” leaves will not allow the parylene vapors to adequately penetrate into the book.
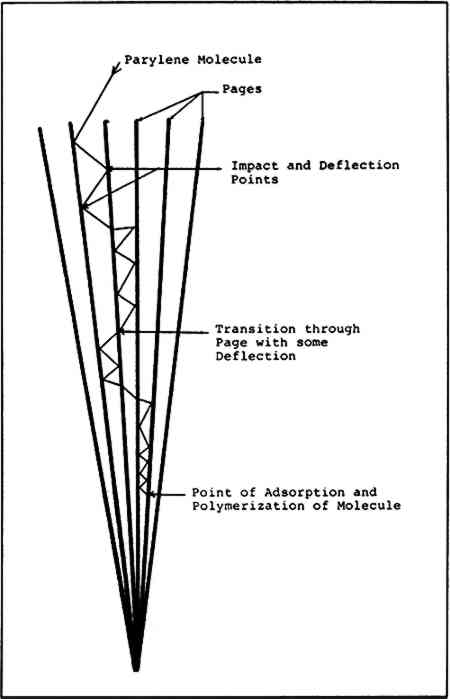
Pumping was continued until a suitable pressure for deposition was reached and then the book was returned to atmospheric pressure and removed from the chamber. The result was some minor warpage of the pages and cover due to moisture loss. The book was then left to equilibrate at ambient humidity in the lab which allowed the book to return to its normal pre-vacuum condition. The amount of Once it was determined that the leaves of a book would arrange themselves to facilitate penetration of the gaseous phase of the polymer, actual attempts at “in situ” polymerization could be considered. 5 A REVIEW OF TECHNICAL PROBLEMS POSED BY VACUUM POLYMERIZATION IN BOUND VOLUMES5.1 Apparent vs Micro-Surface Area of PaperA book measuring 19 � 13 cm which contains 400 numbered pages consists of 200 individual leaves, each of which has an apparent surface area of 494 cm2. The entire book will then have an apparent surface area of almost 102,000 cm2 or a little over 10m2. This includes the covers and protective inner leaves which are not numbered but must be included in the surface area measurements. Apparent surface area, then, is that area which is readily apparent to the unaided eye. The microscopic surface area however, can be several times larger than the apparent surface area depending on the type or composition of the paper being treated. It is this microscopic surface area—consisting of millions of individual cellulose fibers—which is relevant and important when discussing parylene deposition and polymerization. It must be remembered that parylene polymerizes on a molecule by molecule basis. The parylene molecule is approximately 35 � 20 angstroms in size and it is at this level of size that coating and consolidation initiates. Parylene will coat all the surface area available down to this level of scale. What this means is that larger amounts of parylene will be required to coat this micro surface area. It also means that very initimate contact with a substrate will occur. This is one of the reasons for the irreversibility of the process: contact with the substrate is so initimate and on such a minute scale that removal without damage to the paper would be impossible. 5.2 PenetrationThe strengthening of paper which is bound in book form poses a considerable challenge to any consolidant. In an ideal case, the polymer should penetrate and polymerize equally on all parts of a book. Using parylene, it is not possible to accomplish this with the book closed. A book processed in the closed state would become bound on all edges due to “polymer bridging” from one page to another. This would in turn form a barrier to further penetration of the parylene and the desired strengthening would not occur. The book must be processed while open, and the leaves arranged so that there is some interval maintained between each of the leaves to allow entry of the parylene molecules. Once all these criteria are met there is still the problem of “diminishing interleaf spacing.” The distance between any two adjacent leaves might range from .5 to 1.5 mm at the outer edges of the book. This distance diminishes to almost zero at the binding. In order to treat fully a bound volume, parylene molecules must find their way to the bottom of all the multiple crevices created between leaves due to their being bound at one edge. Figure 4 is a diagram illustrating the hypothetical path of a single parylene molecule entering a book and traveling some distance toward the binding before finally adsorbing and polymerizing on the surface of a page. It is interesting to note that parylene molecles will pass between paper fibers with varying ease depending
5.3 Gaseous Diffusion Through PaperThe gaseous diffusion of parylene through paper was tested by sealing test substrates (an aluminum or glass strip) inside cubes made from newsprint and foldur kraft paper. After treatment the cubes were opened and the substrates checked for the 5.4 Polymer Thinning in Bound VolumesAs noted earlier, the parylene molecule is very active. The molecule will bounce off the substrate many times before the proper kinetics and angle of impact occur, resulting in adsorption and subsequent polymerization. The very close spacing of the pages greatly increases the number of “molecular impacts” and hence greatly increases the chances that a molecule will adsorb and polymerize before reaching the inner portions of a book. As a result the coating is thickest at the outer page boundries and grows thinner toward the center binding edge of each page. This halo penetration effect can be seen in Figure 5. The dark area in the center has not been coated due to an insufficient penetration of polymer.
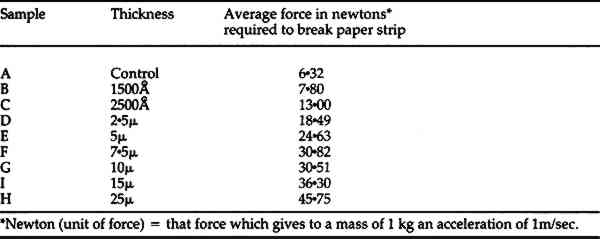
A further explanation for this thinning effect is that polymer which has already adsorbed and polymerized in the outer areas of the book tends to operate as an additional “sink” for the free parylene diradicals still in the gas phase. In simple terms, In order to determine the amount of thinning which might occur at a specific treatment level the following test was conducted. A book was selected and test strips were placed at the edge, middle and binding side of several pages throughout the book. The book was then placed in a chamber and sufficient parylene added to allow for penetration to the binding edge of the pages. After removal from the chamber the test strips were measured. The test strips indicate that the thickness ranges from 2.5 microns at the outer edge to .5 microns near the binding. At this point in the research it appears to be impossible to avoid this gradient in bound books. Uniform coatings of any desired thickness can be obtained by disbinding the book and processing the individual pages on trays 3–5 mm apart—a rather costly procedure. (Fig. 6).
If the gradient remains constant from book to book, which has yet to be demonstrated, the desired thickness could perhaps be extrapolated from readings taken at the outer edge of the pages. The ratio derived from this work appears to be 5:1 in this case. A 5 micron reading at the page edge indicates that 1 micron should coat the paper at or near the binding. It appears that in order to obtain desirable coatings near the binding edge, heavier amounts must be accumulated on the outer periphery of the pages. 6 DETERMINATION OF DIMER CHARGETHE DETERMINATION of how much parylene dimer is needed to charge the system is based on the amount of surface area which is being coated in the depostion zone, and the coating thickness desired. The formula for making these determinations is based upon many years of practical experience in Nova Tran's deposition laboratories. However, most of this data is based upon work with smooth non-porous substrates such as metal, plastics, glass, etc. As discussed in the section on “Technical Problems,” paper contains large amounts of micro surface area. The very large surface areas contained in bound volumes necessitated some modification of existing formulae. It was decided to prepare and test a group of several books in an effort to arrive at a new formula that would apply to bound volumes. The amount of apparent-surface area in each of the books was calculated and an arbitrary amount of parylene charge was assigned to each book. The arbitrary formulae were as follows:
A total of 16 similar books from a series were parylene treated, three books at each level of treatment. Each of the three books at each level was treated with a different form or combination of forms of parylene. Parylene “C” and Parylene “N” are the most commonly used types, and of these “C” is used far more than “N.” Parylene “C” has slightly better moisture barrier properties and a faster deposition rate, while Parylene “N” has a slower deposition rate but greater penetration characteristics. This experiment would thus serve two purposes: (1) determine the correct dimer charge required for any given surface area, and (2) give some indication as to the best parylene type or combination of types which would give the best results. 7 IMMERSION TESTONCE THE BOOKS WERE TREATED with parylene it was necessary to devise a means of rapidly determining the extent of treatment in each volume. Other than a slight change in the “feel” of the paper, it was difficult to tell a parylene treated volume from one that had not been treated. An immersion test was set up whereby each volume was placed upright in a container of water. The pages were “fanned” open as much as possible to insure that the water would penetrate between the pages. Weights were also placed on top of the The books were left in water for periods of up to 4 months. They were checked on a weekly basis for evidence of water damage. Those areas not penetrated by the polymer darkened as the water penetrated them (Fig. 5). The results of these preliminary tests indicate that the best overall coverage and penetration is achieved by using a combination of Parylene “N” and Parylene “C”. The ratio used in this experiment was 1 to 1. Other ratios may have varying results. In this category the best overall results were obtained in books treated at the .005 and .006 amount of parylene. These books showed evidence of penetration and coating down to the binding, the area that is most difficult to reach with polymerization treatments. 7.1 Additional Results of Immersion TestingAs stated earlier, the purpose of the immersion experiment was to determine the proper amount of dimer to use in order totally to treat a bound volume. Some interesting additional conclusions can also be derived from this study. Figures 7 and 8 show treated and untreated volumes after four months of total immersion after which they were removed from the water and placed in plastic boxes while still wet. They were left in the boxes for an additional three months, after which these photographs were taken.
The untreated volume is in very poor condition, perhaps a total loss. The cover was destroyed during the first few weeks of immersion due to dissolution of the glues used in binding. The pages can be separated only with great difficulty. They have the consistency of wet tissue in that they come apart at the slightest touch. The paper is also much darkened in color. By contrast, the treated book has survived intact. The glue has been dissolved but the parylene has acted as a consolidant. Even the gold stamping on the spine has been held in place. The remarkable thing to note is the excellent condition of the pages. Their color is quite natural and they have absorbed little if any water. Most of the resident moisture is surface or interfibrous entrapment, held much like a sponge holds water. The pages are not stuck together and can even be “fanned” with the fingers as though the book were dry. One of the volumes which had been given a heavier parylene treatment was placed in a vacuum oven and baked dry at 90�C. The book came out in very good condition. There was no water spotting or discoloration of the pages nor was there any evident weakening of the paper. It is realized that this is not a standard conservation practice. The object here was to stress the test book and look for evidence of failure on the part of the coating. One other phenomenon noted during the immersion tests was the lack of swelling on the part of parylene treated books. It appears that the hydrophobic properties of the polymer may aid in the prevention of swelling during immersion. No comparison tests were made, nor were any books immersed in the closed state. It would seem reasonable to assume from these tests and observations that parylene treated books would have a better chance to survive flooding or high humidity situations. 8 MOISTURE CONTENT AND DEPOSITION EFFICIENCYSTUDIES WERE CONDUCTED to determine the approximate amount of parylene (by weight) deposited on a book during the coating process. The material adsorbs and polymerizes on any cool surface, so it naturally coats the chamber walls and some is lost down the cold trap. Some of the books treated during the course of the study were subjected to the following analysis. The book was weighed and then placed in the vacuum chamber and evacuated to a point where parylene deposition would normally be initiated (4 – 10 microns). At this point the book was brought back to atmosphere by bleeding of dry nitrogen gas into the chamber. The book was quickly removed from the chamber and weighed. It was then returned to the chamber to which had been added a charge of parylene. The system was then evacuated to the previous level and the parylene was deposited. The chamber was then returned to atmospheric pressure, again using dry nitrogen gas. The book was removed and quickly weighed. The dry nitrogen gas was used to prevent moisture from the air entering through the bleed valve. The difference obtained between the first and second weighing is the amount of water that is lost during the process of evacuating to deposition pressures. The difference between the second and third weighing is a close approximation of the weight gain caused by deposition. The efficiency factor is obtained by dividing the weight gain by the amount of parylene initially placed in the chamber. Some sample results are as follows: Example 1 -
Example 2 -
Tests on several other books showed deposition efficiencies ranging from 45 to 50%. This is a higher rate than is obtained for the usual commercial substrates. It is suspected that this higher efficiency is due to the greater amounts of surface area found in books. The moisture loss incurred during the process averages 3 to 5%. The book will regain this lost moisture, usually in three to five days after removal from the vacuum system, depending on the ambient humidity in the room. The process can be hastened by placing the book in a high humidity environment. All books treated by vacuum processes must have their normal moisture content restored. Some types of paper lose flexibility when subjected to high vacuum and must be handled with care until the moisture content returns to normal. 9 TENSILE STRENGTH AND FOLD ENDURANCESOME TENSILE STRENGTH TESTS were run on random sample pages taken from treated books. The machine used was a Chatillon light capacity universal motorized test stand model LTCM. The results of these tests correlate with the findings on polymer thinning mentioned earlier. The tensile strength of the paper is greatest in the peripheral areas of each page and decreases toward the center binding edge of each page. It should be pointed out that even very thin coatings of parylene will cause the tensile strength of paper to go up. A coating as thin as 2500 angstroms can double the tensile strength. Figure 9 is a chart showing the increase in tensile strength of standard newsprint as the thickness of the parylene coating is increased.
10 FOLD ENDURANCEIT IS THE AUTHOR'S OPINION that fold endurance tests currently used for paper bear little relation to stresses incurred during normal use. Parylene coatings may be progressively applied to achieve any level of fold endurance desired. The paper will fail at the same number of folds as an uncoated control, but the parylene web will hold the two sections together indefinitely. Fold endurance tests at the Library of Congress have demonstrated this phenomenon. It must be noted here that parylene does not chemically interact with paper, or any other substrate for that matter. The material is very inert and the strengthening is purely mechanical in nature. Parylene surrounds and strengthens the individual paper fibers as well as welding the fibers together where they contact one another. The decision that has to be made concerns how much one is willing to spend to achieve this “pseudo fold endurance” created by progressive coating. Some of the books treated by the author were printed on very thick stock (17-18 microns). Treatment with parylene resulted in a significant increase in tensile strength but no increase in fold endurance was noted. The paper still broke after one or two folds. The reason this occurs is due to the fact that the ratio of paper thickness to coating thickness is so It is the author's feeling that protection against simple disintegration should be the objective for most books. This kind of protection is usually attained at thinner coating levels than those required to attain higher levels of fold endurance. In an actual case in point, a brittle book was brought to the author by a local resident who had heard of my research into this problem. The book in question was a farm implement catalog from the 1880s. The pages of the book were very brittle and many had crumbled and eroded edges. He very much wanted something to be done to save the book from complete disintegration, and the author agreed to process the book as part of the research program. After processing it was noted that fold endurance had increased from one fold to break to 5-6 folds to break. The tensile strength appeared to have increased but could not be quantified without destructive testing. The “handleability” of the book was greatly enhanced, to the point where pages could be handled and turned with little danger of further damage. The owner of the book was extremely pleased with the result of this effort. One of the great advantages of this material is the precise degree of control which can be exercised over the deposition of the polymer. While one cannot reverse the process, it is possible to go forward by very minute increments, stopping at each stage to remove the book or object from the chamber to check for the desired result. The reactive sites for “polymerization continuation” will remain open for several hours. Materials returned to the chamber for additional coating after 8-12 hours will still produce continuous film properties on the finished item. 11 GENERAL CONCLUSIONSTHE RESULTS of this initial effort give every indication that the gas phase consolidation of books with the paraxylylenes offers great promise as a potential means of extending the useful life of embrittled and/or weakened books. The author is currently working in cooperation with the Library of Congress staff, processing books for them by the methods outlined in this paper. They are conducting an exhaustive evaluation of these books to determine the efficacy of this process for treating that portion of their collection which has become embrittled. The results of their study will be published in the very near future. Should all the results turn out to be positive, a massive task will still remain. This task will be to scale up the parylene deposition technology to allow for the treatment of hundreds or thousands of books in a single large chamber. The laws that govern the distribution of a gas phase polymer such as parylene would make this a very formidable task. The author also has a cooperative research effort under way with the J. Paul Getty Conservation Institute. This effort will focus on the preservation of ethnographic materials with parylene. The results from these various efforts should provide a wealth of data that is not available at the time of preparation of this introductory paper. In the case of books, the options available to address the large (and still growing) crisis of embrittled books appear to be few in number, to the extent that no potentially viable solution can be ignored. Reversibility will certainly be an issue. The The paraxylyenes, their method of deposition and the results of this study show interesting potential to the extent that they have attracted the attention of major institutions concerned with conservation. Serious investigations are now under way. The author is hopeful that his summary of initial efforts to strengthen books will serve to stimulate further interest and investigation into the potential contribution which this family of polymers can provide to the art and science of conservation. REFERENCESHumphrey, B., “The Application of Parylene Conformal Coating Technology to Archival and Artifact Conservation,” Studies in Conservation, vol. 29, no. 2 (August 1984). BACKGROUND READINGFibrous Materials Coated with Linear Paraxylylene Polymers, U.S. Pat. No. 3, 429, 739, February 25, 1969. The literature cited below refers to para-xylylenes but not to the specific applications described in this paper. Patents granted to Michael Szwarc and Union Carbide Corporation: US Patents 3288728, 2341754, 637651, 650947, 740886, 883939, 973901, 11120422, 1112894, 1428168. Trade literature: “Solvent resistance of the parylenes,” “Custom artifact encapsulation/protection” and “Parylene pellicules for space application” (Nova Tran Corporation); “Parylene removal with oxygen plasmas” and “Parylene conformal coatings” (Union Carbide Corporation). Other publicationsParks, L.R., “Coatings in search of metallurgical applications,” The Metallurgist and Materials Technologist (July 1977): 371. Szwarc, M.S., “Polyparaxylylene; its chemistry and application in coating technology,” Polymer Engineering and Science16 (1976).
 Section Index Section Index |