THE EXAMINATION, TREATMENT AND ANALYSIS OF A PAIR OF BOOTS FROM THE ALEUTIAN ISLANDS INCLUDING A NOTE ABOUT POSSIBLE PESTICIDE CONTAMINATIONAnn Boulton
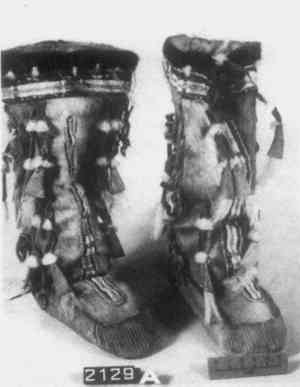
ABSTRACT—During the course of the examination and treatment of a pair of boots from the Aleutian Islands, the author was confronted with problems regarding materials identification. Lack of suitable hair identification keys and possible contamination of pigments by pesticides are discussed. Identification of hair and pigments is reported. Use of cast paper pulp as a filling material for seal skin is described in the treatment section. 1 INTRODUCTIONA PAIR OF CHILDREN'S BOOTS from the Aleutian Islands (NMNH cat. #2129; Fig. 1) was examined and treated while the author was an intern from the Art Conservation Department, State University of New York College at Buffalo. The work was done at the Anthropology Conservation Laboratory of the National Museum of Natural History (NMNH), Smithsonian Institution. The boots were collected by the U. S. Naval Exploring Expedition of 1838–1841 led by Lt. Charles Wilkes. They were added to the Smithsonian's collection in 1857 when all of the material collected by Wilkes was transferred from the U. S. Patent Office.
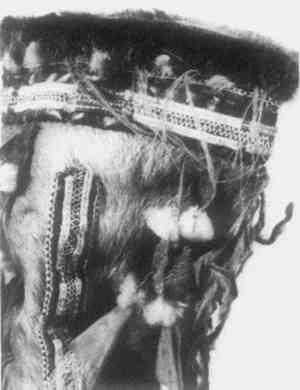
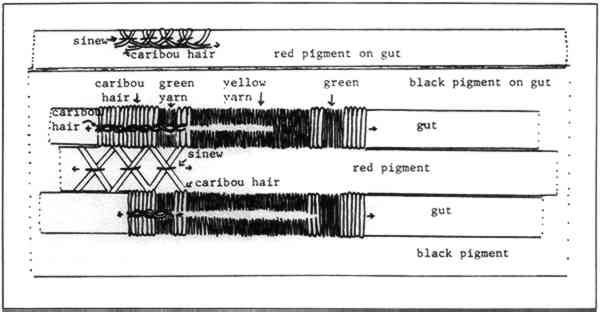
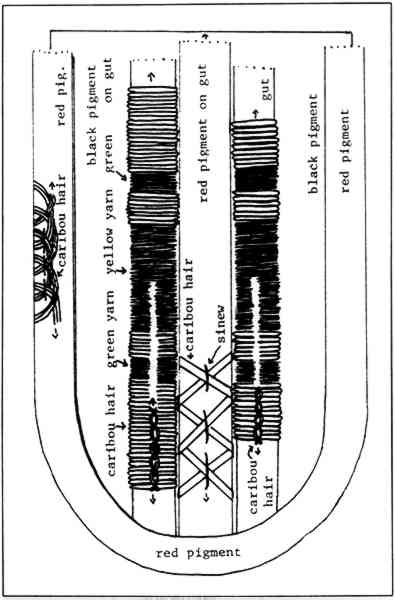
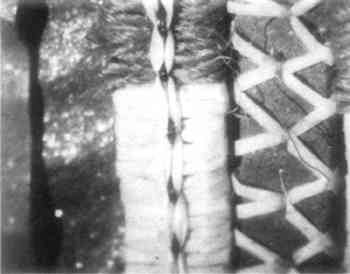
2 PROCEDURES2.1 DescriptionThe boots are 23cm in height and 10.2cm in diameter at the top. The upper portion of each boot is composed of caribou calf skin with fur attached, and the lower portion is of seal skin (see section 2 for identification). The top is trimmed with what was later identified as seal skin with fur, the ends of which have been clipped. Just under this edging, an intricate band of gut appliqu� with white caribou hair embroidery encircles the boot. Two similar bands of embroidered gut appliqu�, attached with sinew, run down the front and back of the boots (Fig. 2). Much of the gut is covered with pigment and is thus difficult to characterize. In some small areas, however, where the pigment has been lost, the gut is translucent and may be seal esophagus.1 In the center of this band is a strip of gut covered by red pigment, found to be red ocher, and overlaid with caribou hair embroidery. On either side of the red strip fine green and yellow wool yarns are used in conjunction with caribou hair to create a striped pattern. This was achieved by wrapping the yarn and hair around gut strips and then, with sinew, attaching the strips adjacent to the red strip. Another piece of esophagus, covered with a layer of glittering black pigment, underlies and extends beyond the yarn-wrapped and ocher-covered strips to create a banded effect (Figs. 3 and 4). Between the top seal skin edging and the embroidered band described above hang sparse, long, wispy threads of yellow wool interspersed with long wispy white hairs, probably polar bear, and short tufts of very fine white hair, possibly ermine.
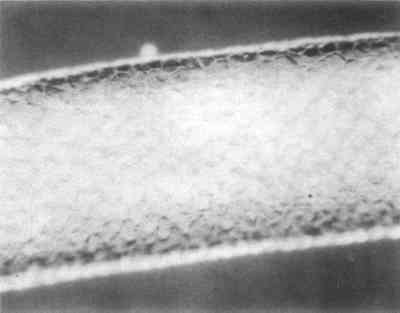
The boots were identified as Eskimo on the catalog card; however, an article on hair embroidery describes in great detail caribou hair embroidery similar to that found on the boots and pinpoints the Aleutian Islands as the place of origin.2 Although Turner states that caribou were not indigenous to these islands, and their fur was obtained through trade,3 Murie reports that in primitive times Barren Ground Caribou (Rangifer arcticus) were more plentiful than today on the Aleutian Island of Unimak.4 This confusion may stem from the statement that reindeer, also called Woodland Caribou (Rangifer caribou), were not native to the Aleutians but were introduced some years ago on the islands of Umnak and Atka.5 Caribou skin along the side seams of the upper portions has been left long and cut into fringe. Additional fringe has been attached randomly with sinew in six other spots on the upper portion of each boot. All fringe has been colored red with what appears to be red ocher. Two tufts of fine white hair, possible ermine, are attached with sinew to each added fringe. The end of each fringe is enclosed by a flattened bird beak, and long white hairs, possibly polar bear, extend from the bottom of each beak. The soles are made of seal skin which has had all of the hair removed except for a small patch. This skin is attached to the upper part of the boot with sinew, and the toe and heel are finely gathered. 2.2 Materials IdentificationAn attempt was made to identify all of the hair. First, a list of fur-bearing animals indigenous to Alaska and the Aleutians was compiled. An identification key for fur of the Arctic could not be found and keys to other geographic areas were of limited use. Next, examination of the hair in situ in reflected light with low magnification (up to 40x) and examination of mounted samples in polarized light at higher magnification was conducted. Scale casts were made in a variety of media such as gelatin, PVA-AYAC (Union Carbide) and Polaroid print coater. The casts did not prove to be very useful as most of the hairs examined had partially lost their scales The caribou and seal fur are the most distinctive. Caribou guard hair is characterized by its hollow center, round cross section and opacity. When viewed in reflected light at low magnification its surface appears pitted, much like an orange peel (Fig. 5). Because of its hollow and brittle nature, it often has collapsed or flattened areas. Mountain Goat, Mule Deer and Moose hair also have similar characteristics. The hairs used for the embroidery are relatively large in diameter and are said to come from the bell of the caribou, that is, the area under the neck which is white and ruff-like (Fig. 6).6 The caribou fur used for the main body of the boots is much finer in diameter and is brown and white.
Seal guard hair is characterized by its nearly flat cross section. Each hair is narrow at the proximal end, widens in the middle and tapers to a fine flat point at the distal end (Fig. 7). In reflected light at low magnification, the hairs appear shiny and translucent, as if wet. The only other animal of the Arctic with hair of similar appearance is the otter. Otter and seal fur can be differentiated in that the narrowness of the proximal end of a seal hair is only a small portion of the overall length of the shaft, while otter hair exhibits a narrow proximal end for about half its length before widening to its maximum diameter. No attempt was made to pinpoint the species of the seal.
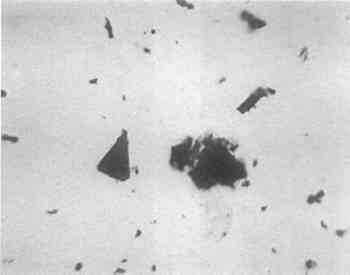
The polar bear guard hair is distinctive because of its white color and its length, which is in excess of 12.5cm on these boots. The only arctic animals with white hair matching the length of that of the polar bear are the caribou and man. The distinction between caribou and polar bear hair is easily made because polar bear hair, when observed in reflected light at low magnification, is transparent and the medulla is easily seen through the transparent cortex. In addition, it is much finer in diameter than caribou and the transparency gives it a glistening appearance resembling that of glass wool. White human hair also exhibits this glistening transparency; however, the medulla is not continuous and in some hairs is practically nonexistent. The medulla is less than a third of the total diameter of the hair shaft. The medulla of the polar bear hair is much more substantial; it has no breaks and is at least half the diameter of the shaft. The extremely fine short tufts of white fur identified as ermine above is the least positive of the several identifications. Ermine is a name sometimes given to a species of weasel also know as the Shorttail Weasel (Mustela erminea). It is dark brown with white under parts in summer and entirely white only in winter.7 Other possibilities are the Least Weasel (Mustela rixosa) and the Tundra Hare (Lepus othus), both of which have very fine hair which turns white in winter. Although not all of these animals are found on all of the Aleutian Islands, it is reasonable to assume that if caribou skins was obtained by trade, other skins could have been procured in the same manner. Additional comparisons should be made at a future date. The black pigment on the applique gut substrate was also investigated (Fig. 5). Its smooth application, rough texture and sparkling appearance closely resembled that of fine silicon carbide sandpaper; it was initially thought to be fish skin and not recognized as a pigment layer. Close visual inspection indicated that it was pigment, and a sample examined under polarized light was found to be opaque, fibrous and isotropic—in short, charcoal-like and interspersed with translucent anisotropic red triangular platelets. The unknown was separated mechanically by color into black and red components. The particles which appeared to be red and translucent in transmitted light were found to be an opaque metallic blue-gray in reflected light and undoubtedly the source of the sparkling appearance of the black pigment. A paint with a silvery glitter used on the Aleutian island of Unalaska is mentioned as face adornment, and the use of hematite as a red pigment in the Aleutians is mentioned by Hrdlicka.8 (Hrdlicka has an excellent compilation of primary source material regarding pigments used by the Aleuts.) Hematite also exists in a harder compact rhombohedral form which may be cut for jewelry and is not red, but a lustrous silvery gray.9 The metallic blue-gray portion of the unknown was compared with a known sample of hematite with the same glittering appearance and was found to be similar in polarized light. Both exhibited bright red translucent triangular platelets (Fig. 8)
References to Eskimo use of gunpowder, plumbago and charcoal as black pigments, and blood as a binder are made by Nelson.10 Coal is said to have been a source of black pigment on Nunivak Island.11 Nelson's reference to the use of gunpowder as a pigment provoked a comparison of the black pigment with a sample of gunpowder. Nineteenth-century black gunpowder is primarily charcoal with sulfur and potassium nitrate. The black pigment appeared to be charcoal, that is, opaque, fibrous and isotropic between crossed polars, and the gunpowder compared favorably. A second comparison was done with a scanning electron microscope. The structures of the black pigment and the gunpowder appeared similar though not identical and not particularly distinctive. Interestingly, neither appeared to be particularly
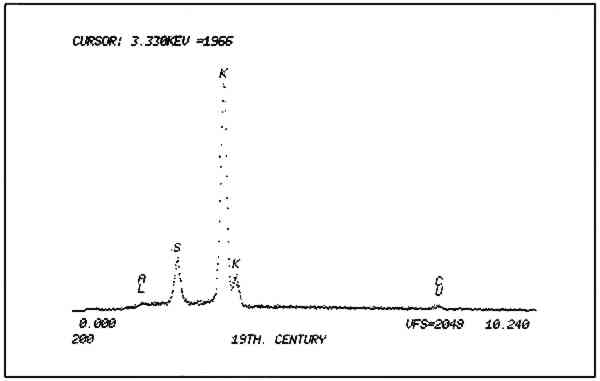
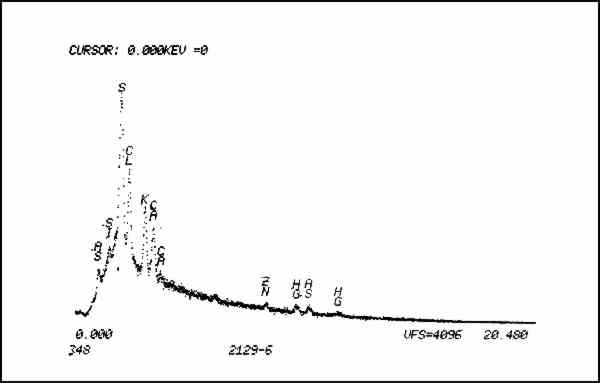
Non-quantitative energy dispersive x-ray analysis was then performed on both samples by Walter Brown, National Museum of Natural History, using a Cambridge Stereoscan 250 MK2. As expected, the gunpowder yielded peaks for sulfur and potassium (Fig. 10). The unknown, however, contained, sulfur, chlorine, potassium, silica, calcium, iron, mercury, and arsenic (Fig. 11).
The presence of arsenic was the clue that contamination of the specimen might have occurred from the application of pesticides while in the museum's collection. This pair of boots was collected about 1840 and has been part of the Smithsonian's holdings since the museum's founding. Arsenic is known to have been widely used as a pesticide in the museum, as has mercuric chloride, called Corrosive Sublimate by Walter Hough, an archeologist at the U.S. National Museum, forerunner of the National Museum of Natural History.12 (Other pesticides containing chlorides and sulfides which have either documented use in the museum or are suspected to have been used because of their popularity for natural history collections are: ethylene dichloride, carbon tetrachloride, arsenic sulfide, paradichlorobenzene, dichlorodiphenyltrichloroethane (DDT) and carbon disulphide.) The potassium found in the unknown could be attributed to the use of saltpeter (potassium nitrate) and potash (potassium carbonate), both of which were sometimes mixed with arsenic trioxide which was used as a solution applied to skins.13 The calcium may be due to the mixing of precipitated chalk with white arsenic (arsenic trioxide) which was applied as a powder on fur skins. The presence of iron in the unknown is attributable to the hematite used for the sparkling effect. Analysis of a discreet particle of the suspected hematite yielded iron peaks alone. Lastly, the silica is probably due to the presence of dirt. In light of the fact that the boots are insect damaged, it is not unreasonable to assume that they were treated at some point with any number of pesticides which the charcoal of the pigment would have readily adsorbed. Therefore, contamination of the specimen by pesticides has effectively prevented any determination of the identity of the pigment except on morphological grounds. The black pigment is charcoal; whether this charcoal originally included potassium nitrate and sulfur which gunpowder would have contained cannot be determined. The red pigment (Fig. 5) was examined in polarized light and compares favorably with known samples of red ocher. The yellow and green fibers used in the embroidery were determined to be wool by polarized light microscopy. 2.3 ConditionThe boots were stiff, distorted and partially filled with old packing material made of wood shavings (excelsior). Two wooden forms about � inches thick and cut in the rough shape of the boots filled the feet. The lower portion of the proper left boot 2.4 Treatment
3 CONCLUSIONIF THE USE OF THE PESTICIDES had been documented, perhaps further information could have been obtained regarding the use of gunpowder as a pigment. This is yet another There is a need for the development of hair identification keys to be developed which are useful to conservators. Most keys which exist are meant for use by biologists or wildlife enforcement personnel and usually assume certain information which is unavailable to the conservator, such as size and feeding habits, location of the fur on the animal, etc. Without these facts the flow charts of existing keys often cannot be followed. Brown's “Microscopy of Mammalian Hair for Anthropologists,” the only key found which is intended for artifacts, is not useful as a flow chart because it is restricted to carnivorous animals. There is nothing particularly distinctive about carnivore hair which sets it apart from that of herbivores as a group. The only way to categorize animal hair in key form which makes any practical sense is by geographic area. Usually, though not always, this information is known to the conservator. Another objection from the conservator's point of view is that, in order to use these It is the author's opinion that a key could be created for hair of the arctic from observations made in reflected light at magnifications below 40x which could make distinctions, for example, between groups such as caribou and seal. The obvious advantage to such a key is that sampling would no longer be necessary; the hair could be examined in situ. Identification of specific species, of course, could be accomplished through use of polarized light, higher magnification and scale casts. However, such distinctions may be of greater interest to biologists than conservators. As mentioned above, scale casts are of no value if the hair is worn or otherwise deteriorated, as is often the case with older anthropological materials. Color is also an unreliable criterion because of the possibility of changes caused by light, tanning chemicals and pesticides. In addition, tremendous color variation occurs in some species. The ideal key for the conservator should avoid using information obtained from these sources as much as possible. As with any attempt at materials identification, a reference collection of known samples is the most indispensable tool. ACKNOWLEDGEMENTSTHE AUTHOR WOULD LIKE TO THANK Carolyn Rose, Michele Austin, Edith Dietze, Donna Strahan and Cathy Valentour of the Anthropology Conservation Laboratory, NMNH, for their assistance and support; Frank Greenwell and Catharine Hawks of the Division of Mammals, NMNH, for access to the mammals collection and information on mammal specimen contamination; Dr. Alfred Gardner of the Department of Interior, for assistance in hair identification; Walter Brown of the SEM Laboratory, NMNH, for pigment analysis; and Martin Burke of the Division of Conservation, National Museum of American History, for providing gunpowder samples. This project could not have been done without their help. The author also gratefully acknowledges financial support for the internship from the Andrew W. Mellon Foundation through a fellowship awarded by the Art Conservation Department, SUCB. REFERENCESTurner, p. 35. Ibid. Ibid., p. 44. Murie, p. 331. Ibid., p. 332. Turner, p. 35. Burt, p. 56. Hrdlicka, p. 102. Webster, p. 217. Nelson, p. 198. Ray, p. 20. Hough, p. 558. Ibid., p. 555. Ibid., p. 553.
Clark, p. 64. BIBLIOGRAPHYAdorjan, A.S. and Kolenosky, G.B.A Manual for the Identification of Hairs of Selected Ontario Mammals. Ontario Department of Lands and Forests, Research Report (Wildlife) No. 90, 1969 Appleyard, H.M.Guide to the identification of Animal Fibers. Leeds: Wool Industries Research Association (WIRA), 1960 Black, Lydia T.Aleut Art. Anchorage: Aleutian/Pribilof Islands Assn., Inc., 1982 Brown, Martin F. “The Microscopy of Mammalian Hair for Anthropologists.” Proceedings of the American Philosophical Society. 85 (1982): 250–274. Burt, W.H. and Grossenheider, R.P.A Field Guide to the Mammals. Boston: Houghton Mifflin Co., 1976 Clark, Thurid. “Conservation of an Aboriginal Wallaby Skin Water Bag at the Australian Museum.” Recent Advances in Leather Conservation, SonjaFogle, ed.Washington: Foundation of the American Institute for Conservation of Historic and Artistic Works, 1985. Hall, E.R. and Kelson, K.R.The Mammals of North America. New York: The Ronald Press, Co., 1959. Hicks, John W.Microscopy of Animal Hairs, A Practical Guide and Manual. Washington: Federal Bureau of Investigation, 1977. Hough, Walter. “The Preservation of Museum Specimens from Insects and the Effects of Dampness.” Annual Report of the Board of Regents of the Smithsonian Institution 1887. Part 2. Washington: Government Printing Office, 1889, pp. 549–558. Hrdlicka, Ales. The Aleutian and Commander Islands and Their Inhabitants. Philadelphia: Wistar Institute of Anatomy and Biology, 1945. Moore, Tommy, D., Spence, Liter E., Dugnolle, Charles E.Identification of the Dorsal Guard Hairs of Some Mammals of Wyoming. Cheyenne: Wyoming Game and Fish Department Bulletin no. 14, 1974. Murie, Olaus J.Fauna of the Aleutian Islands and Alaska Peninsula. Washington: U.S. Fish and Wildlife Service, 1959. Nelson, Edward. “The Eskimo about the Bering Strait.” 18th Annual Report of the Bureau of American Ethnology 1986–97. Washington (improved reprint, Smithsonian Institution Press, 1983), pp. 3–518. Pence, R.J.Analyzing Fur Damage with a Microscope. Circular of California Agricultural Experimental Stations Extention Service, 541 (1966): 1–18. Ray, Dorothy Jean. Aleut and Eskimo Art. Seattle: University of Wasington Press, 1981. Turner, Geoffrey. Hair Embroidery in Siberia and North America. (Occasional Paper on Technology,7. Pitt Rivers Museum, University of Oxford.) Oxford: University Press, 1955. Turner, Lucien M.Contributions to the Natural History of Alaska. Washington: Government Printing Office, 1886. Webster, Robert. Gems, Their Sources, Descriptions and Identification. vol. 1. London: Butterworths, 1962. Wildman, A.B.The Microscopy of Animal Textile Fibers. Leeds: Wool Industries Research Association (WIRA), 1954. Williamson, V. H.H. “Determination of Hair by Impressions.” Journal of Mammalogy32 (1951): 81–84.
 Section Index Section Index |