THE QUANTITATIVE TESTING AND COMPARISONS OF PEEL AND LAP/SHEAR FOR LASCAUX 360 H.V. AND BEVA 371Kenneth B. Katz
ABSTRACT—The experiment involved the quantitative testing of bond strengths produced by the reactivation of dried adhesive, Lascaux 360 H. V. and Beva 371. Reactivation was by heat or solvent activation (xylene). These reactivation methods were chosen because of the apparent new interest in lower temperature linings and linings that can be easily reversed, mechanically. Bond strengths were also tested and compared to canvases that were sized with 10% B 72. Results indicate that sizing the original canvas can increase the peel strengths significantly during heat activation. It also appears that solvent activation of the both adhesives produce stronger peel strengths than the heat activated samples. The results corroborate empirical tests and are meant only as broad guidelines for using these adhesives in the ways tested. 1 INTRODUCTIONTHE TECHNOLOGIES OF LINING PICTURES to auxiliary supports appear to be headed away from penetrating adhesives, towards nonpenetrating adhesives with lower heat activations or no heat activation at all. Most comparisons and “testing” have been individual efforts using intuitive assessments to evaluate the results. Gustav Berger began quantitative tests (lap shear and peel) of adhesives specific to conservation in the 60's,1 and the latest results of Alan Phenix and Gerry Hedley2 produce more relevent data. Empirically, in the laboratory, I have seen that the bond strengths produced by heat activation of Lascaux 360 H. V. were less than solvent activation of the same adhesive. It also appeared that these strengths were in the same ranges as flock nap bonds of Beva 371.3 Furthermore, I had seen the impregnation of original canvas with B-724 done routinely in Italy when performing cold linings, and wanted to see if that affected the strengths of the bonds produced. The purpose, therefore, of my testing was to measure and record the results of lap/shear and peel tests ofof a relatively new adhesive, Lascaux 360 H. V. and compare it to the bond strengths of BEVA 371 when the two adhesives were used in relatively the same manner, that is, when the adhesives were solvent activated or heat activated. Sizing on the reverser of the original canvas was performed on half the samples to see the effect sizing had on the strengths of the bonds. 2 EXPERIMENTTHE SUPPORT USED AS THE ORIGINAL canvas was a light-weight Belgian linen. The Belgian linen was stretched, wetted, and restretched again. After the second stretching, Liquitex gesso was applied to simulate a ground. A few days later, two coats of Rohm and Haas Paraloid B-72 (10% in xylene) were brushed into half the sample canvases. (The application made the linen noticeably stiffer.) The lining fabric in all cases was a woven plyester, P&S #39. The first experiment involved the application of Allois K. Diethelm AG Lascaux Acrylic adhesive 360 H. V. to the lining support. The adhesive was used undiluted from its container and applied through a fiberglas window screen material using a squeegee. After a 24-hour drying time, a second coat was applied in the same manner. The samples were lined to produce a 1″ overlap for shear/lap testing and 3″ overlap for peel testing. Five samples were lined for each category and labeled. The labels were as follows: To summarize the above table; each category had 5 test strips, 1″ wide, which would be tested. “P” stood for peel test, “H” stood for heat activation, “U” stood for the plain grounded Belgian linen. In another example: “L” stood for the samples intended for lap/shear testing, “X” for xylene activation and “S” for the grounded Belgian linen that had been sized with the B-72. The linings were executed 15 days after application of the Lascaux 360 H. V. The heat activation of the Lascaux 360 H. V. took place on a Convectron vacuum hot table using (.001mm) Mylar as a membrane. The vacuum, as registered on the gauge, was never more than 2″ of Hg and the top of the table measured 58�C (138�F)∗ The temperature remained at 58�C for 5 minutes before the table was set on “cool.” The samples were rolled once using a nappy paint roller. The samples were on the table for a total of 45 minutes. Solvent activation of the Lascaux 360 H. V. took place using a paper suction table and xylene. The samples were placed on blotters which were placed on the suction table. Xylene was brushed over the adhesive and 30 seconds later, when the adhesive was tacky, the Belgian linen was placed on top. A Mylar membrane was used to cover the whole table surface and the suction table was run for 45 minutes.
∗All temperatures were recorded using a digital temperature meter. See footnote 3. The next material tested was Adam Chemical Company's BEVA 371. The same original support and lining fabrics as used with the Lascaux 360 H. V. were used with the BEVA 371 samples. A one-quart spray container was � filled with un-diluted BEVA 371. The metal container was placed in a hot water bath and stirred until the adhesive became clear. Xylene was poured into the adhesive (⅓ by volume). Sprayer pressure was set at 50 lbs/sq. inch and the BEVA 371 was “flocked” by spraying onto the lining fabric to a thickness of about 3/32″ (Fig. 1)
The heat-activated samples using Beva 371 as an adhesive were lined using a Versavac portable suction table and Dartek membrane. The vacuum was 30″ H20 (2.16Hg) and the temperature of the adhesive was 60�C. The solvent activation was also performed on the Versavac. Xylene was brushed onto the flocked BEVA 371 until it was translucent and tacky. As with the Lascaux 360, the original canvas samples were then placed on the lining fabrics. The samples were then covered with a Dartek membrane and put under 60″ of H20 pressure. After 1� hours, the membrane was removed locally, keeping the airflow on, to allow the BEVA to dry. A strong solvent smell was still noticeable and no adhesion was noted. The samples remained on the table with just airflow, without a membrane for another � hour and were then allowed to air-dry over night. No weights were used. The overnight evaporation of the solvent produced markedly stronger bonds. The samples were cut into the 1″ wide strips and labeled as follows: All samples were sent to the Nordson Corporation testing facility in Westlake, Ohio where lap/shear and peel tests were performed on an Instron 1115 tester. The conditions were as follows for the test:
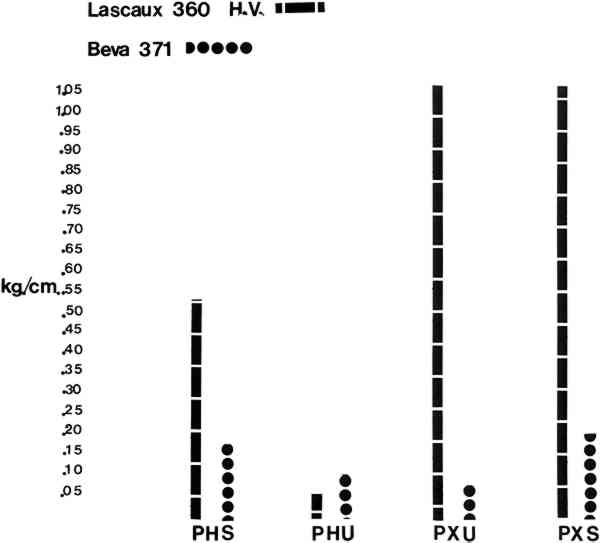
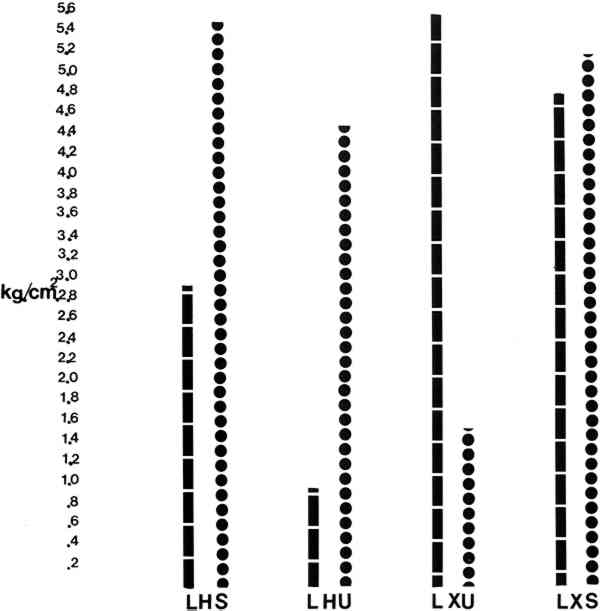
Results: (see Tables/Fig. 2, 3) all samples were climatized to 72� F and 55% RH Table 2 Comparisons of Average Peel Test Strengths Between Lascaux 360 and Beva 371 Table 3 Summary and Comparisons of Lap/Shear Tests Between Lascaux 360 and Beva 371
3 REPORTING OF THE DATATHE DATA FOR THE PEEL TESTS were calculated by averaging the results from each of the five test pulls. In the case of the MAXIMUM values, the highest peak on each run was chosen and the figure which appears in Table 1 reflects the average of those five peaks as well as the standard deviation. In the case of the MINIMUM values listed, the lowest points on each of the five runs was chosen and the figures seen reflect those averages Table 1 Summary of Data for Peel Tests of Lascaux 360 and Beva 371 The lap/shear results were obtained by recording the peaks on each of the five runs and then averaging them together. The standard deviations were also calculated. In the discussion which follows, the AVERAGE values are used as comparisons in interpreting the results. Table 1 is printed to present the standard deviations for those that are interested. 4 DISCUSSION:THE VALUE OF 300g/2.5cm has been offered as the minimum acceptable value for a lining by Phenix & Hedley. For the discussion which follows, 300g/2.5cm has been reduced to .115kg/cm for easier comparisons. Table 2 and Figure 2 include the results obtained by peel testing the Lascaux 360 H. V. and BEVA 371. By comparing average values, one sees that the Lascaux 360 H. V. exhibits higher peel strengths than BEVA 371 in all applications except PHU (heat activation of unsized canvas). One also sees that the xylene activation of Lascaux 360 H. V. (PXU, PXS) produces the highest peel values, almost 10 times the Phenix & Hedley parameter. When the xylene-activated samples (PXU, PXS) are compared to the values recorded for heat activation (PHS, PHU), one also sees that the qualitative results corroborate the intuitive and empirical tests which first instigated this experiment, namely, that xylene activation of Lascaux 360 H. V. produces a stronger bond than heat activation of the same adhesive. When one looks at the effects of sizing the original canvas with 10% B-72, one sees that in all cases of heat activation, the sizing increases peel strength. In the case of the Lascaux 360 H. V. one sees almost a 10-fold increase in peel strength, from PHU 360 (.059kg/cm) to PHS 360 (.546kg/cm). In this case, sizing the original puts the value well over the Phenix & Hedley value of .115kg/cm. On the other hand, it would appear that heat activating a canvas not sized with B-72 would not produce a bond strong enough to hold down cupping or extreme distortions. In the case of the BEVA 371, sizing the original canvas with B-72 almost doubles the value for peel strength from PHU 371 (.100kg/cm) to PHS 371 (.180kg/cm). This again would put the adhesive into the Phenix & Hedley range. Table 3 and Figure 3 compares the Lap/Shear tests. One sees on graph that the relative values coincide with the peel tests of each adhesive, respectively. However, it is very interesting to note that the values of Lap/Shear are consistently higher for BEVA 371 than Lascaux 360 H. V., except for LXU 371 and LXU 360. The data from Tables 1, 2, 3 would indicate that BEVA exhibits a relatively low peel strength and relatively high shear strength. It should also be noted that the values for the lap/shear test are a bit Table 4 tests the relative amounts of adhesive left on the original canvas after mechanically peeling the lining canvas and original apart (as resulted from the experiment). It is clear that BEVA 371 consistently remains on the lining canvas, where it should be, and is not really affected by the B-72 sizing. On the other hand, one sees that especially in the xylene activation of Lascaux 360 H. V. residues can be a problem. One also sees that sizing with B-72 increases the probability of residue transfer. When one compares the values between PHS and PHU one sees a slight increase in residue transfer, as does one when comparing the values between PXU and PXS. Table 4 Amount of Adhesive Remaining on Original Canvas (0 = No Residues … 5 = Uniform Amount of Total Surface) What do these numbers really mean? First, it would appear that sizing an original canvas with B-72 increases peel strength and bonding when low temperature activation is used; in the case of BEVA 371, almost 2 times, in the case of Lascaux 360 H. V., 10 times. Second, xylene activation of Lascaux 360 H. V. produces the strongest bonds of any methods or materials tested. Third, mechanical reversibility may be determined by bond peel strength. One sees that the BEVA 371 remains consistently on the lining canvas as it should, but its peel strengths are relatively low. When one looks at the residue data for Lascaux 360, the lower peel strengths produce the same results as the Beva (no residues); but the higher values of Lascaux indicate a problem in mechanical reversibility. The data may also indicate that the B-72 sizing may increase the amount of residue when Lascaux 360 H. V. is xylene activated. In conclusion, the numbers give some insight into handling these two adhesives in the low heat level modes, or solvent modes, but a word of caution is needed. The data from this experiment gives relative strengths which Phenix & Hedley continue to establish by their ongoing research into peel and lap/shear testing. However, from a practical point of view, one has to test these techniques/applications personally to get a feel for the bonds produced. For example, it is my personal experience after peeling all the samples that even though BEVA 371 (PHU & PHS) have relatively low values and fall below Phenix & Hedley's .115kg/cm or hover around it, they still produce a strong enough bond for the ‘nap’ bond lining system for which they were intended. Of course it might not hold down cupping or severe distortion, but when used as intended by Lodge and Albano it appears to produce acceptable bonds. On the other hand, my experience with Lascaux 360 PHU indicates that the bond is slightly weaker than the ‘nap’ bond and an average figure of .059kg/cm should be considered a relatively weak bond strength. Furthermore, in speaking with colleagues, it appears that there are many variables at work affecting the strength of the bonds, including time between application and use of the adhesive, the using of a space blanket during lining, pressure used, and, in some cases, the amount of time between lining and testing. Some people report that the Lascaux 360 H. V. bond gets stronger with time. These results are presented in the spirit of Phenix & Hedley to begin to quantify the empirical findings and give some broad insights into handling these materials. ACKNOWLEDGEMENTSBetty L. Baxter and Margaret A. Salo for their total cooperation and assistance in typing and word processing this article; Dr. Walter Cobbs for permission to use the Nordson facilities; Most of all, Mr. William Rehman of The Nordson Corporation, whose cooperation and insights made this experiment possible. MATERIALS:P & S Textiles, Jordan Rd., Skaneateles Falls, NY 13153 Terry Polyester #39Conservation Materials Ltd., 240 Freeport Blvd., Box 2884, Sparks, NV, 89431 Lascaux 360 H. V. Acrylic AdhesiveAdam Chemical Co., 18 Spring Hill Terrace, Spring Valley, NY 10977 Beva 371 AdhesiveTalas, 213 W. 35th Street, N.Y.C., NY 10011, 1 � 2 Belgian Linen Plain weave, 8 threads in warp, 15 threads in weftREFERENCESBerger, G., Testing Adhesives for the Consolidation of Paintings. Report sumbitted to Kress Foundation1969, pg. 37 PhenixAlan; Hedley, Gerry, Lining Without Heat or Moisture. ICOM Committee for Conservation, 7th Triannual Meeting, Copenhagen1984 Albano, A.Lodge, R.AIC Painting Specialty Group, Milwaukee, May 1982. “Critical Nap Bond Temperature Monitoring—Synthetic Fabric and Adhesive Application in the Lining of a Klee and Leger.” Rohm & Haas Paraloid B-72: Copolymer of ethyl acrylate and methyl/methacrylate.
 Section Index Section Index |