PROTECTIVE SURFACE COATINGS FOR DAGUERREOTYPESM. Susan Barger, A.P. Giri, William B. White, William S. Ginell, & Frank Preusser
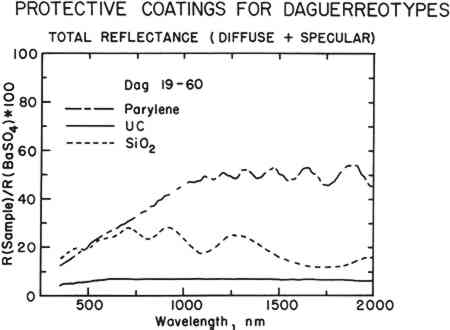
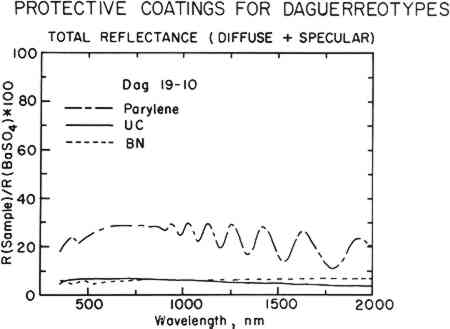
3 RESULTS AND DISCUSSIONOF THE FIVE SPUTTERED COATINGS, two, SiO2 and BN, produced coatings of interest. TiO2 and AIN were not acceptable because the sputtered coatings were non-stoichiometric. Although in principle these materials have desirable optical features, the sputtered films did not match the optical properties of the bulk materials. LaB6 produced a metallic, absorbing film that gave the daguerreotypes the appearance of detailed tintypes. Because LaB6 is a metal, this is not surprising. However, it was of interest to test a metal-on-metal coating to see if it would severely affect the daguerreotype appearance. In addition, TiO2 and LaB6 both exhibited poor adhesion to the daguerreotype surface. These coatings were excluded from further study, and for that reason no micrographs or spectra of the materials are included. However, these samples are included in Table 4 which provides a subjective description of the appearance of all the daguerreotypes studied. The failure of the TiO2 coatings was due, primarily, to the non-stoichiometry of the sputtered films. Pure TiO2 would have been an acceptable coating material. However, even small deviations from stoichiometry (one or two parts per thousand) produce a material with high electrical conductivity and strong optical absorption. Clearly, this would not be a desirable coating. Our coating procedure produced TiO2-x, where x varied between ∼0.1–0.4. All of these coatings were strongly colored and tended to overwhelm the image. The coating structure was inconsistent and had poor adhesion to the daguerreotype surface. The coatings of AIN were very consistent and the film structure closely mimicked the underlying daguerreotype. Unfortunately, all of these coatings were bright pink due to non-stoichiometry of the film. Decreasing the film thickness only reduced the intensity of the pink coloration. The AIN coating maintained the specularity characteristic Silica was sputtered successfully to produce coatings of SiO2. The SiO2 films had a pinkish-to-greenish cast, but the coloration was not pronounced. The films retained the specular, metallic appearance of uncoated daguerreotypes. A typical set of reflectance spectra that illustrate the difference between the uncoated and SiO2-coated portions of one of the sample daguerreotypes is shown in Fig. 1 (Dag 19–60). Ideally, the visible spectrum of a coated daguerreotype should match that of an uncoated sample. Mismatches indicate that the optical properties of the daguerreotype have been altered because of the coating. The degree of matching is directly related to the appearance of the coated daguerreotype. The short-dashed line in Fig. 1 shows the typical reflectance spectrum of an SiO2 film on a daguerreotype surface. The regular undulations in the spectrum are due to interference fringes. Generally, an SiO2 film on a daguerreotype surface is slightly more absorbing than an uncoated daguerreotype.
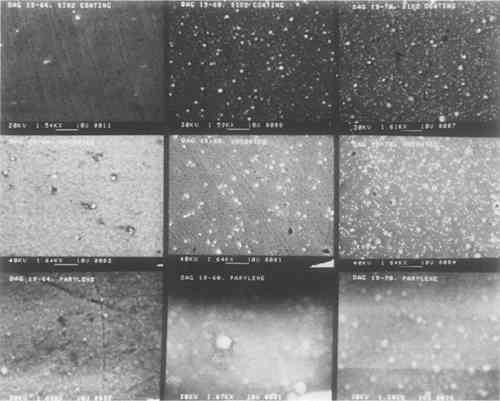
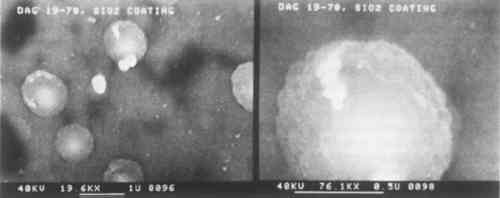
The SiO2 coatings also have a shimmery appearance which comes about because of their film structure. SiO2 tends to form sheets of small silica spheres whose diameters are less than 1 μm. The micrographs in the top row of Fig. 2 show the general sphere structure of these coatings and the changes with film thickness. The SiO2 film shown for sample Dag 19–64 does not show the spherical structure because of the deposition conditions.11 The thickest film has the highest spherical SiO2 particle density. This structure is typical of silica, and it is what gives rise to the fire in gem opals. Figure 3 shows individual SiO2 spheres at two high magnifications. Surprisingly, even though these SiO2 spheres are about the same size as daguerreotype image particles,
The sphere-like structure does not provide a continuous coating for the daguerreotype. Spaces between the spheres are likely places for the initiation of corrosion. This effect was demonstrated in the accelerated aging tests described below. BN forms a consistently dense, continuous film that closely follows the underlying daguerreotype surface, as seen in the first row of micrographs in Fig. 4. Thicker films tend to be the most dense. These coatings compare favorably with the uncoated portions of the sample daguerreotypes (middle row of Fig. 4). Although BN is usually formed with a graphite-like hexagonal structure (i.e., soft) and is susceptible to moisture attack, proper control of the sputtering conditions produes a very hard, diamondlike BN phase which, not surprisingly, has many of the properties of diamond, i.e., optical transparency, resistance to chemical attack, and high hardness.12 The preparation of this metastable cubic BN phase has just recently been discovered and has a number of potential applications, including coatings on cutting tools.
Freshly deposited BN coatings have a gold or rose-gold cast. As the coating ages, this coloration tends to dissipate and the films become colorless. This change in
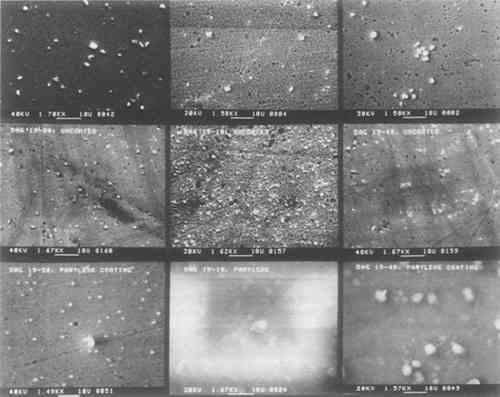
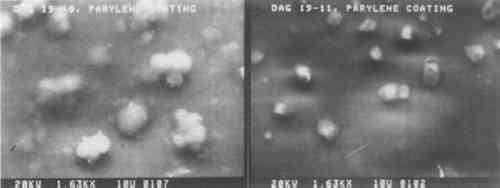
One portion of each of the fifteen daguerreotype samples used in this study was coated with Parylene C. This coating is transparent and colorless, but there are noticeable interference fringes on many of the Parylene C-coated samples. However, their occurrence and severity may be controlled by altering the coating thickness. Unlike any of the other coating materials, Parylene C may be removed using orthodichlorobenzene. The structure of the Parylene C films varied from sample to sample. Some coatings have many clumps of unreacted dimer material distributed over the film surface, as seen in the left micrograph (Dag 19–18) of Fig. 6. A few of the Parylene C films were totally structureless and appear to be draped over the image microstructure. This is shown in the right micrograph (Dag 19–11) of Fig. 6. These two micrographs show the extremes of Parylene C film structure. Other Parylene C films are shown in the bottom rows of micrographs in Figs. 2 and 4. Some of the micrographs appear indistinct because of interactions between the non-conducting polymer and the electron beam of the SEM. These films were not gold coated prior to SEM examination
Parylene C films are more absorbing than any of the other coatings, primarily because they are from 5 to 40 times the thickness of the sputtered films. The long-and-short dashed lines in the spectra of Figs. 1 and 5 show typical reflectance data for these films. The undulations in the curves are due to interference fringes. All of the Parylene C films have similar spectra, although some coatings are less absorbing than others because of film thickness variation among the samples. Most of the Parylene C films reduce the specularity of the daguerreotype and cause the surface of the image to appear dulled or matte. The image remains visible, but there is a loss of contrast and detail. In some cases, the image tone was shifted from a “warm” to a “cold” cast. The image contrast appears almost reversed (positive to negative) in the most severe cases. Thus, while the image is still visible, the characteristic image quality associated with daguerreotypes is lost to a large extent. Daguerreotypes are usually cased and the case provides a fairly effective barrier to corrosion. Unlike most other silver objects, cased daguerreotypes are more vulnerable to oxidation rather than to sulfidation. Some silver sulfide is present on daguerreotype surfaces, but the prevalent corrosion product is silver oxide. This is because of the vastly greater availability of oxygen in the general environment over the availability of unreduced sulfur, as well as to the physical constraints of the traditional daguerreotype package. Fluctuations of the environment within the daguerreotype package, such as changes in water vapor (humidity), temperature, and air exchange, control the corrosion rate. The size of the environment inside the package also affects corrosion. The presence of small amounts of harmful materials can have a greater effect because there is only negligible dilution from the outside atmosphere. For these reasons, the environment within the package can be quite harmful to the daguerreotype. A preliminary test of the ability of BN, SiO2, and Parylene C films to provide protection against corrosion of daguerreotypes was carried out using sulfur-containing gases. These tests were carried out in sulfur gas atmospheres because the kinetics of sulfur attack of silver are much faster than those of oxygen attack. Thus, in a relatively short time, these tests give a very good qualitative clue as to the protective characteristics of the coatings. This is particularly true with regard to mechanical completeness or freedom from pinholes and flaws. These tests are not very effective for accessing the resistance of the films to corrosion initiated by diffusion of gases through the film (unless the transport mechanism for sulfur or oxygen is the same for a given film). They also give very little information about chemical attack of the film itself. Since it is expected that daguerreotypes will always be sealed in cases, these last two points are of minor interest compared to the completeness of the films. Samples of BN and SiO2-coated unimaged daguerreotype plates were subjected to gaseous H2S (vapor over a saturated aqueous K2Sx solution) and SO2 (vapor over a 0.5 M NaHSO3 solution) in separate sealed beakers over a 72-hour period. A Parylene C-coated daguerreotype (19–79) was exposed only to the H2S environment. Uncoated areas on the samples served as controls. The concentrations of H2S and SO2 were not measured, but these exposure conditions are orders of magnitude more severe than would be expected during normal storage of daguerreotypes. Within one hour, the uncoated controls showed evidence of corrosion. At the end of the test, most of the BN-coated plates and the Parylene C coated sample were unaffected, but the SiO2 films did not prove protective. The mechanical adhesion of the BN and Parylene C coatings to the daguerreotype surface was tested using Method B described in the ASTM Standard D3359-83, Standard Methods for Measuring Adhesion by Tape Test. This is a very simple test used to establish adequate adhesion of coating materials to metal substrates. A razor blade is used to score the coating; a piece of transparent tape is placed over the scored area and burnished using the eraser end of a pencil; finally, the tape is pulled off the surface. The scored area is inspected under a light microscope for removal or peeling of the coating. The tests for both the BN and Parylene C showed either no peeling or removal (Classification 5B). This is good evidence of the strong adhesion of these films to the daguerreotype surface. The long-term stability and aging properties of the coatings were not examined. Although all organic materials oxidize and are subject to accelerated degradation in the presence of light, antioxidizers and stabilizers are available that will prolong the useful life of polymeric coatings. Tests have demonstrated that the generic Parylenes have excellent oxidation resistance which can be enhanced further by incorporation of selective antioxidants.13 However, should degradation occur, the coating is reversible and may be replaced. The sputtered film materials were chosen because they are inert and should not interact with either the environment or the daguerreotype over the course of time. However, these films cannot be easily removed if they fail. It would be possible to sputter the coating off the daguerreotype surface, but this may not always be desirable. If the sputtered coatings are used, they should be considered a permanent addition to the daguerreotype. |