PRESSURE-SENSITIVE TAPE AND TECHNIQUES FOR ITS REMOVAL FROM PAPERMerrily A. Smith, Norvell M. M. Jones, Susan L. Page, & Marian Peck Dirda
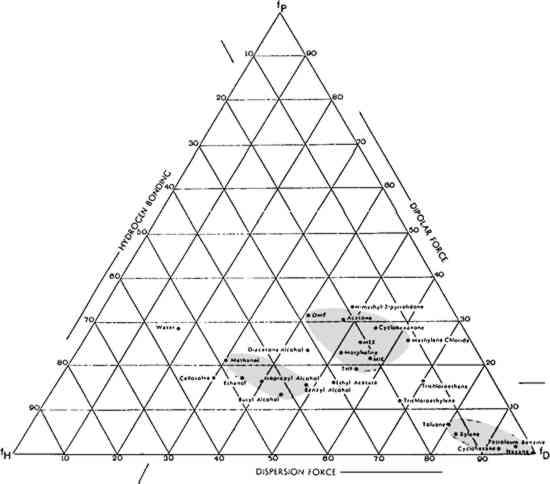
3 SOLVENT SELECTION AND TESTING3.1 SelectionFinding an effective solvent for a given pressure-sensitive tape is based largely on trial-and-error procedures in combination with knowledge gained from previous experience. The Teas chart of fractional solubility parameters (Fig. 2) is useful in identifying effective solvents because it enables testing for solubility in a systematic manner. It also enables the conservator to make educated guesses about which solvents to mix to achieve specific results. The Teas chart plots solvents according to their three components of molecular interaction: polar forces, hydrogen bonding, and dispersion.
The chart in Figure 2 shows the location of a number of solvents commonly used in paper conservation. Three general groups of solvents have been circled: aliphatic and aromatic hydrocarbons (lower right); ketones and miscellaneous solvents (center); and alcohols (left). When conservators refer to “strong” solvents, they often loosely equate strength with what is, in fact, high polarity; and this common practice is followed in this paper. It so happens that polar solvents such as acetone are more likely to dissolve natural resins, printing inks, and many paper dyes than less polar solvents. Acetone is a strong solvent for these important materials; by extension, it has come to be thought of as simply a “strong” solvent. Continuing with this non-scientific, but empirically useful ranking, the non-polar aliphatic hydrocarbons (hexane) are the weakest solvents, followed by the aromatic hydrocarbons (xylene, toluene), then the ketones and miscellaneous solvents (methylene chloride, acetone), and ending with the strong, polar alcohols. Increased polarity does not necessarily result in increased solubility, however. The most effective solvent in a given situation is the one whose solubility parameter matches the solubility parameter of the materials to be dissolved. One does not need a chart to recognize this solvent because it will be, by observation, the one that works best. For example, acetone, normally considered a “strong” solvent, does not dissolve wax, whereas hexane, normally considered “weak” does. For wax, then, hexane is a stronger solvent than acetone. |