PRESSURE-SENSITIVE TAPE AND TECHNIQUES FOR ITS REMOVAL FROM PAPERMerrily A. Smith, Norvell M. M. Jones, Susan L. Page, & Marian Peck Dirda
ABSTRACT—The history of the development of pressure-sensitive tape is outlined from its invention in 1845 to the present. The aging properties of different tapes are described. The efficacy and testing of various organic solvents are examined with regard to appropriate solvent selection for tape removal. Specific techniques for the removal of tape by immersion, poultice, and suction are discussed. 1 HISTORYPRESSURE-SENSITIVE TAPE—so-called because light pressure causes it to stick readily to most surfaces—typically consists of four component layers (Fig. 1). Two of these layers are readily recognizable: the adhesive mass, which is usually composed of a synthetic or natural rubber (more recently of an acrylic polymer), and which may contain a variety of softeners, antioxidants, plasticizers, and curing agents; and the backing, or carrier, which may be foil, crepe paper, fabric, cellophane, cellulose acetate, plasticized polyvinyl chloride, or any of a number of other flexible materials, and which may be reinforced with glass or other fibers. Less apparent but equally important are the keying coat, or primer, used between the adhesive and backing to insure good adhesion between the two (it may be based on natural or synthetic elastomers and may contain some tackifiers); and the release coat, applied to the side of the backing that is away from the adhesive mass, so the roll can be unwound without leaving any residual adhesive. Details about the nature of the adhesive mass and the backing are generally obtainable from the manufacturer, but components of the keying coat and release coat are proprietary information.
Pressure-sensitive tape was first developed in 1845 by Dr. Horace Day, a surgeon, who devised a method of applying a natural rubber adhesive to strips of cloth, thus producing a kind of surgical tape which he used in his practice.1 The next major application for a pressure-sensitive tape came from the auto industry in the 1920s. Two-toned automobiles were becoming increasingly popular, and manufacturers needed an efficient way to produce a clean, sharp edge where two From this beginning the pressure-sensitive adhesive industry began to grow. New tapes were developed and new applications for them were found. Gradually, the adhesive component was changed to synthetic rubber compounds, and a new transparent backing material was developed from regenerated cellulose, called cellophane. In the 1950s cellulose acetate and its copolymers came into use as tape backings, and synthetic polymers combined with resins came into use as adhesives. Among the first of these tapes was Scotch Brand #810 Magic Mending Tape, a matte-surface (frosty) tape that consists of a cellulose acetate carrier and an acrylic polymer adhesive. As the development of tapes progressed and people recognized the convenience of using tape to mount, hang, and mend almost anything that came to hand, questions began to arise about the stability, removability, and long-range effects of the materials involved. In the 1970s—partly in response to demands from the conservation, picture-framing, and library communities—two companies started producing so-called “archival” pressure-sensitive tapes: Hans Neschen International developed Filmoplast P and Filmoplast P90; and later in the decade Ademco introduced Archival Aids Document Repair Tape. Filmoplast P has a relatively transparent short-fibered acid-free paper carrier, which now (but not initially) contains a calcium carbonate buffer. Filmoplast P90 has a heavier paper carrier, also buffered. The adhesive on both tapes is an acrylic ester that is laid on from a water-based dispersion. The Archival Aids Document Repair Tape also has a paper carrier that, according to the manufaturer, is made from acid-free, sulphur-free bleached wood pulp and a butyl-acrylate acrylic adhesive that contains a small amount of dibutyl-phthalate plasticizer to give it a pressure-sensitive characteristic. The growth of the adhesive-tape industry in less than sixty years is nothing short of amazing: 3M alone manufactures 1,000 different kinds of tape that will stick just about anything to anything. The conservator's lot seems to be how to get them unstuck. 2 AGINGUNTIL RECENTLY, the tapes most frequently encountered on archival materials and art on paper were masking tape and cellophane tape. Both have rubber-based adhesive. As these adhesives oxidize, they pass through distinct stages of deterioration, the exact nature of which has been documented in studies conducted by Robert Feller.3 Initially, there is a period of little alteration, an induction time. During the induction time, removal is relatively easy. As oxidation progresses, this stage is followed by a fairly abrupt change in adhesive consistency and color. The adhesive mass gets very sticky and oily, perhaps, in part, because of the breaking up of the rubber polymer. It also starts to yellow. Apparently during this stage various components of the adhesive soak into the paper, rendering it translucent. Some components probably remain, at least temporarily, on the surface. In this oily condition the adhesive mass can penetrate the paper entirely and move into adjacent sheets. Its components can also begin to affect certain media—particularly printing, typing, and ballpoint pen inks—causing them to The adhesive, having permeated the paper, continues to oxidize, and gradually loses its adhesive properties. The carrier may fall off, and the adhesive residues crosslink, becoming hard, brittle, and highly discolored. Once it has reached this condition, the adhesive residue and the stain it has created are very difficult, sometimes impossible, to remove. The aging process differs in the new acrylic adhesive tapes. They don't discolor appreciably. The adhesive mass does not typically soak into the paper as rubber-based adhesives do. The acrylic adhesive is, however, subject to cold flow and will penetrate to the degree that paper porosity allows. According to 3M literature, this difference in aging behavior may occur because each acrylic adhesive is one homogeneous polymer which, once coated to the backing in its final form, is pre-crosslinked. Because of this pre-crosslinking the acrylic pressure-sensitive adhesives (with the possible exception of those used in the so-called “archival” tapes) are not soluble in any of the solvents used in paper conservation. They can only be swollen and scraped or brushed off mechanically. The initial reaction to the advent of Scotch Brand #810 Magic Mending Tape was one of great excitement. In 1961 3M summarized the tape's characteristics as follows:
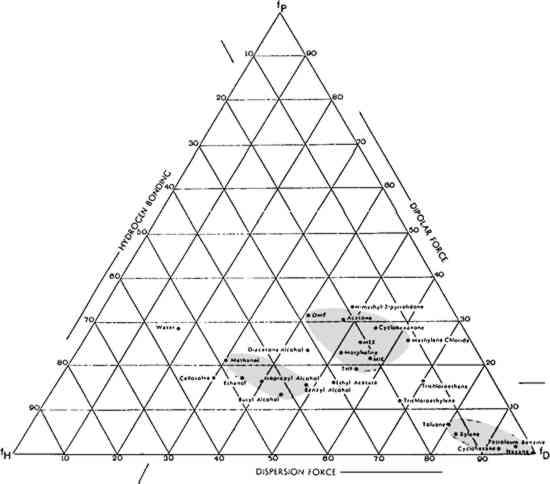
It is worth remembering that the composition and formulae of proprietary products may be altered by manufacturers, with no corresponding change in either trade name or packaging. Magic Mending Tape is no exception. A newly purchased roll of the tape in the Library of Congress Conservation Office was lighter in color than an older roll, which was noticeably more yellow. In the absence of contrary information, one might think that the yellower roll had deteriorated. In fact, according to 3M, the formulation of the tape had been changed. Also, optical changes may have occurred due to shrinkage in the roll resulting from storage conditions. Because of the possibility of formula changes in manufactured products, it is unwise to automatically apply testing data from a roll of tape manufactured in 1983, for example, to one manufactured in 1984. Nevertheless, we have now had an opportunity to observe the behavior of some acrylic tapes as they age naturally. We have seen slight penetration in some papers. We have also observed some discoloration of tape and substrate on groundwood paper. A piece of acrylic frosty tape has been observed to cause bleeding of red ballpoint pen ink 3 SOLVENT SELECTION AND TESTING3.1 SelectionFinding an effective solvent for a given pressure-sensitive tape is based largely on trial-and-error procedures in combination with knowledge gained from previous experience. The Teas chart of fractional solubility parameters (Fig. 2) is useful in identifying effective solvents because it enables testing for solubility in a systematic manner. It also enables the conservator to make educated guesses about which solvents to mix to achieve specific results. The Teas chart plots solvents according to their three components of molecular interaction: polar forces, hydrogen bonding, and dispersion.
The chart in Figure 2 shows the location of a number of solvents commonly used in paper conservation. Three general groups of solvents have been circled: aliphatic and aromatic hydrocarbons (lower right); ketones and miscellaneous solvents (center); and alcohols (left). When conservators refer to “strong” solvents, they often loosely equate strength with what is, in fact, high polarity; and this common practice is followed in this paper. It so happens that polar solvents such as acetone are more likely to dissolve natural resins, printing inks, and many paper dyes than less polar solvents. Acetone is a strong solvent for these important materials; by extension, it has come to be thought of as simply a “strong” solvent. Continuing with this non-scientific, but empirically useful ranking, the non-polar aliphatic hydrocarbons (hexane) are the weakest solvents, followed by the aromatic hydrocarbons (xylene, toluene), then the ketones and miscellaneous solvents (methylene chloride, acetone), and ending with the strong, polar alcohols. Increased polarity does not necessarily result in increased solubility, however. The most effective solvent in a given situation is the one whose solubility parameter matches the solubility parameter of the materials to be dissolved. One does not need a chart to recognize this solvent because it will be, by observation, the one that works best. For example, acetone, normally considered a “strong” solvent, does not dissolve wax, whereas hexane, normally considered “weak” does. For wax, then, hexane is a stronger solvent than acetone. 4 EFFICACY OF VARIOUS SOLVENTS4.1 Archival TapesFor a short time following their application, Filmoplast P and Filmoplast P90 can be removed with water. After aging, they can be removed only with an organic solvent. Even with organic solvents, the adhesive on these tapes can only be swollen, not dissolved. In samples subjected to accelerated aging tests at the Library of Congress, both tapes could be removed, with difficulty, in xylene, toluene, or acetone. Filmoplast P could be removed more easily than Filmoplast P90. For a short time following its application, Archival Aids Document Repair Tape was removable in mild solvents such as cyclohexane. After accelerated aging, the adhesive on test samples was soluble in xylene or toluene. 4.2 Magic Mending TapeMagic Mending Tape can be released from a fairly hard-surfaced paper with minimal disruption of the adhesive layer by using ethyl alcohol. Toluene, or a mixture of toluene and ethyl acetate, works well to swell the adhesive, which can then be removed by scraping. 3M has suggested a combination of heated toluene and isopropyl alcohol, which seems too dangerous to use. Solvents such as acetone will dissolve the cellulose acetate carrier and drive it into the paper. 4.3 Masking TapeFresh masking tape can be removed with petroleum benzine and toluene; the latter is also effective on tape that has begun to oxidize. Acetone, tetrahydrofuran, and N,N, dimethylformamide may be necessary for badly crosslinked adhesive. Surprisingly, very hot water will sometimes break up surface residues of the crosslinked adhesive. 4.4 Cellophane TapeDuring the induction period, hexane, cyclohexane, petroleum benzine, and ethyl alcohol release cellophane tape. As oxidation proceeds, stronger solvents, such as a mixture of cyclohexane and toluene, or toluene alone, may be necessary. The removal of yellowed, crosslinked adhesive requires methyl ethyl ketone, acetone, methylene chloride, tetrahydrofuran, N,N, dimethylformamide, and sometimes ethyl alcohol for its release. 5 TESTING BEFORE INITIATING TAPE REMOVALSOLUBILITY TESTING with solvents presents difficult problems. Their low surface tension allows solvents to wick rapidly into the paper. A too liberal application of solvent will bleed very soluble inks, colors, paper dyes, or sizes. On the other hand, because solvents tend to evaporate quickly, a sufficient quantity of liquid must be used to get meaningful results. Finally, because solvents are toxic, testing must be done in the fume hood, where the draft compounds the evaporation problem and often no microscope is available. The first step in testing should be carried out on the paper itself in a non-image area. This step is critical because many papers, especially contemporary ones, contain sizes, dyes, or fillers that will move in some solvents. The following series of steps constitutes one possible procedure for testing paper.
Repetition of the process at least three times in the same area is imperative to approximate the effects of the relatively large amount of solvent actually used in tape removal. Particular care in testing is required if the adhesive is to be removed with a poultice because of the lengthy exposure to solvent this technique involves. Some papers form yellow rings with all of the solvents that are likely to remove the tape. In such cases select the solvent that causes the least movement, then use it cautiously. The second step is to test inks and colors. As with paper, repeated testing in the same spot is necessary because media that appear stable initially may move unexpectedly and quickly with prolonged exposure to the solvent. Just because an ink is stable for five minutes in an acetone bath does not mean that it will remain stable for six minutes. Media testing might be approached as follows:
6 METHODS OF TAPE REMOVALTHE CONSERVATOR SHOULD be familiar with a variety of tape-removal methods—immersion, poultice, rolling, suction table—so that the most appropriate one for a given artwork can be selected. In addition, the safest and most effective treatment may involve the use of several techniques on the same object. Flexibility and the use of the many available alternatives, then, are as important in tape removal as in any aspect of conservation. 6.1 The Tape ItselfIt seems preferable to take adhesive tape off paper by first removing the carrier, then removing the adhesive layer. Removal of the carrier can be accomplished either mechanically or with solvents. Mechanical techniques are preferred, where possible, because they expose the entire adhesive layer and enable uniform access to it during subsequent operations in which solvents may be involved. One effective method of separating the carrier from the adhesive mechanically is to place the paper on a hard, flat surface, grasp a microspatula so the blade is parallel to the place of the tape, and gently work the edge of the blade under the carrier. One must proceed slowly, as aggressive movement will result in skinning of the paper. Sometimes the carrier will lift more easily if forward motion of the spatula is combined with a very slight waggling of the blade. Other techniques for mechanical removal have been suggested that involve the use of dry ice, of a hand-held heat gun, or of a tacking iron, or shaving with a scalpel. If mechanical removal of the adhesive carrier is not feasible, and immersion is not contemplated, the carrier can be separated from the adhesive layer with solvents. A mild solvent is painted on the verso of the paper leaf in the area that bears the tape. The solvent penetrates the paper and swells the adhesive layer. With the paper turned so the tape is face up, the carrier is lifted away from the softened adhesive with tweezers. At the same time, more solvent is painted between the carrier and adhesive layer along the peel line. Once the carrier has been removed, as much as possible of the residual adhesive layer remaining on the surface of the paper should also be removed. If the paper surface is hard, the most efficient technique is to remove the adhesive from the nondesign areas with a crepe square, often described as a “rubber cement pick-up square.” It is useful to drag the crepe off the edge of the artwork onto a blotter. The sticky adhesive picks up the blotter fibers, and the whole mass is periodically cleaned off the crepe so the adhesive does not redeposit on the artwork. 7 IMMERSIONIN IMMERSION TREATMENT the entire object is submerged in a bath of solvent, which acts upon the tape to remove the carrier if it has not been previously removed, the surface adhesive, and any resins that have migrated into the paper. The proper choice of solvent allows the conservator to selectively remove the undesirable tape and adhesive without altering other parts of the object. Immersion offers several advantages. It is usually quicker and more efficient than other methods. In fact, speed and timing are very important to its success. It is often gentler than other methods, since the object is subjected to less manipulation, and nothing is added other than volatile solvents. The danger of surface scrubbing is largely avoided. Because of the large amount of solvent used, tide marks are much less of a problem, and stubborn adhesive residues may often be more effectively removed than with other techniques. Finally, apprehension about the long-term aging of a sheet treated locally and thus, perhaps, locally modified in its aging characteristics, is not a factor. Immersion is ideal for single leaves of small to moderate size that have no solvent-soluble components in either the pigments, inks, pencil, ownership stamps, or paper, including dyes, sizes and fillers. When large quantities of tape are involved, immersion is certainly the treatment of choice wherever possible. Immersion is in appropriate for bound volumes and for items with solvent-soluble components and should not be used where a fume hood is unavailable. 7.1 EquipmentThe technique of immersion follows a series of simple steps. First, solubilities are determined by using the local procedures already described, and the best-suited solvent is identified. Next, the equipment and supplies needed for treatment are assembled at the fume hood. An effective, disposable tray may be made from a sheet of polyester film (such as Mylar or Melinex) with the edges folded up and fastened at the four corners with staples. When long soaking time is anticipated, a stainless steel or enamel tray is preferable. The tray should be sufficiently larger than the item to facilitate handling, but small enough to minimize solvent use. Polyester support sheets (either non-woven fiber such as Hollytex or Pellon, or film) should be cut slightly larger than the item, and a polyester film sheet cut to cover the bath if the solvent is a fast evaporator. In addition to small lifting and scraping tools, the conservator will need cotton swabs, a brush, blotters slightly larger than the item, and a stack of 3″ � 5″ blotters. The solvent chosen for treatment should be available in sufficient quantities for several baths, held in a container that can be lifted comfortably. Last but not least, appropriate safety equipment should be used, including goggles, gloves not affected by the solvent, and a standby respirator in case it is necessary to work with one's head in the hood. With all the necessary equipment assembled one is ready to begin. If possible, the tape carrier has already been removed by using the procedures described earlier. 7.2 ProcedurePour solvent into the tray approximately �″ deep. Quickly slide the object into the bath. Hesitation can result in tide marks. Watch the item and manipulate the tray by rocking gently. After a few seconds, if the carrier has not been previously removed, try a corner with a tool. If it moves easily, lift it away. If not, wait a few seconds and try again. Remove the carrier as soon as possible. With luck, any remaining residue will be swollen and soft. If there is suspicion of residue, tip the tray to expose the adhesive mass and search for it in raking light. If there is residue, scrape it gently or roll with a swab. This should be done as soon as possible. Sometimes, especially with precrosslinked acrylic tape, the longer the residue swells and softens, the greater the chance that soft polymer will be trapped in the paper structure and subsequently be more difficult to remove. Acrylic adhesive scraped from the object must be transferred to waste blotter so that it cannot redeposit elsewhere on the object being treated. When the residue appears to be gone, quickly lift the object on its support from the tray to a waiting blotter. Blot quickly. Be wary of the tape area. Most of the adhesive should have been removed, but it may be sticky still. Lift off the top blotter and look for any differential evaporation or sheen revealing remaining residue. If residue is still present, repeat baths as needed. Rubber-based adhesive may need longer soaking times and may require several changes of bath. Sometimes a subsequent bath in a different solvent will diminish a stubborn stain. For example, if the carrier and surface adhesive come off in toluene, the crosslinked resins deep in the paper may respond to acetone. 7.3 RisksImmersion treatment is not without risk, and one should be aware of what can go wrong. Inks that were stable during testing may start to move in the course of treatment. Correction in this case requires judgment. Sometimes it is safest to pull the item from the bath immediately. Other times it is best to wait until the movement has gone to completion and to keep the tray rocking gently. When the color stops moving, then pull the item. The latter approach is appropriate when the bleeding dye is a minor component whose loss does not significantly change the body tone or intensity of the ink. Introduction and removal of the item from the solvent must be quick, even, and complete or tide marks may form which won't move later. Too strong a draft may take the item up the hood as it dries. Watch closely, or anchor a piece of polyester web over the surface during drying. One final warning: Before starting an immersion treatment go over the steps carefully and gather equipment for any possible occurrence. Once the treatment is underway, the conservator cannot safely turn away or leave until the treatment is finished. 8 POULTICETHE USE OF A POULTICE is quite simple. It is a relatively old-fashioned technique and one which we could not do without. A poultice consists of a solvent to dissolve the adhesive and an absorbent. The absorbent can be selected from a wide variety of materials, such as clays (Fuller's earth), siliceous materials (diatomaceous earth or fumed siliceous materials (diatomaceous earth or fumed silica), or cellulosic materials (cellulose powder, filter paper, or torn-up blotters). These two components work like a mud pack. The absorbent prevents the solvent from evaporating too fast while keeping it close to the surface of the adhesive. This allows the adhesive to take on solvent and 8.1 ProcedureDry clean the artifact in the traditional fashion by using vinyl or powdered drafting erasers. Next, mechanically or with a solvent, remove the tape carrier and as much adhesive as possible. Test the adhesive mass remaining on the object to identify the solvents that will remove it. Then test the paper and all nearby inks, dyes, and pigments for their solubility in these solvents. Finally, select the most effective absorbent by experimenting on a test strip of adhesive. Fuller's earth is most frequently selected for objects treated in the Conservation Office. To prepare for poultice applications place the object in a fume hood on top of a sheet of five mil polyester film. Not only does the underlying film focus evaporation of the solvent upward, it also aids in after-treatment clean up by providing a surface onto which the used poultices can be swept. Build a one-half inch powdery dam of Fuller's earth around the outside perimeter of the adhesive. Place additional Fuller's earth in a beaker and mix it with the solvent of choice. The proportion of solid to liquid that seems to work best is slightly more than three parts Fuller's earth to two parts solvent. The resulting poultice should be relatively dry. A dry poultice works better and results in less lateral movement of the solvent than does a more liquid poultice. A poultice that is too dry will not adhere to the paper well enough to draw the adhesive out. Spread the solvent-rich poultice on the adhesive area and allow it to dry thoroughly. To insure that the adhesive is fully extracted from the paper, make sure all solvent has evaporated away from the interface of Fuller's earth and paper before the poultice is removed. When the poultice is dry brush it away. To determine how adhesive removal is progressing, brush a quickly-evaporating solvent such as methylene chloride over the entire area of adhesive residue. The solvent will evaporate from the paper more slowly where adhesive remains. Repeat the entire poultice procedure several times if necessary. 8.2 Advantages and DisadvantagesLocalization of solvent application, the swelling time needed for solvation, and the capillary action that extracts the dissolved adhesive from the paper make the poultice a very effective means of removing pressure-sensitive tape adhesives from paper. Another advantage of the poultice technique is that the rate of solvent evaporation can be altered by changing the environment around the poultice. For example, the rate of evaporation can be slowed by building a polyester tent over the poultice, by putting weights on top of the poultice, or by mounding up additional poultice material. The poultice does have disadvantages. One is that poultice debris can be caught in crevices of porous paper. This debris is especially apparent against dark paper or pigments. One way to remove trapped debris is to rub the area with a soft vinyl eraser or a kneadable eraser. Another disadvantage is that lateral movement of solvent and solvent-carried adhesive can occur, creating tidelines in the paper. The formation of tidelines can be avoided if the amount of liquid in the poultice is reduced. If a drier poultice is used and tidelines still persist, gently swab them with a solvent. The vacuum suction table can be helpful in this process; and, in fact, a combination of treatments is often necessary and more beneficial than a single method. 9 SUCTION TABLETHE SUCTION TABLE or suction disk consists of a flat porous surface, sealed on top of a plenum chamber that is connected to a vacuum pump. The reduced pressure below the surface allows liquids to be drawn through an artwork placed on top. Soluble stains and adhesives can be removed in the process. The major advantage of removing pressure-sensitive tape by suction is that suction limits solvent spread. If the tape lies over solvent-soluble design, the conservator can work around, and so not bleed, the fugitive color. Many papers, especially those of the 19th and 20th centuries, contain solvent-soluble dyes and sizing agents. Control of solvent spread helps to prevent disfiguring rings and stains caused by movement of those components in the paper. Difficulty arises when the solvents that remove the tape also move color in the paper—creating tide lines of liberated dyes and sizing. One solution is to employ two solvents sequentially: a strong one to dissolve the adhesive, and a weaker, misable solvent to flush the stronger solvent and solubilized adhesive from the paper. Employing the restraining solvent can reduce the amount of the strong solvent needed and help localize its effects. The weaker solvent could be any solvent with no or little adverse effect on components of the paper or media. In practice, the strong solvent is applied to the adhesive, the area ringed with the weaker solvent, and then the center flushed with the weak solvent to carry dissolved material into the underlying blotter. Another advantage of the use of suction is that much less mechanical action is involved, than occurs with other local removal techniques, such as rolling with cotton swabs. Thus, abrading solvent-sensitized inks and crushing the paper surface texture are avoided. A typical situation in which suction is useful is characterized by the following example. A cartoon drawing executed in graphite and red pencils had small pieces of cellophane tape lying on the front over both types of pencil. The carrier was still present and adherred to the paper; the adhesive had partially sunk into the paper, causing translucent stains on the reverse, but had reached the hard, gold, crusty stage in only a few places. Tests with solvents that might be expected to remove the adhesive residues caused no movement of sizes, fillers, or dyes in the paper. The graphite was extremely friable, and even the mechanical action of a dry swab was enough to lift it. The red pencil was very soluble in toluene. Given this sensitivity of the image, no removal technique except suction could be used on this drawing. In actual treatment, the carrier was removed locally by painting a mild solvent on the verso and lifting the cellophane with tweezers. Sticky surface adhesive was removed from non-design areas with a crepe square. Then the solubility of the adhesive residues was tested. In this case, toluene dissolved the oily residue within the paper and acetone dissolved the crusty residue. 9.1 EquipmentThe suction table used for many years at the Library of Congress was built in the library and consists of a small fritted plastic top supported by a channeled aluminum base, and connected to a wet/dry shop vacuum. Other designs for homemade suction tables and disks (small, fritted-glass surfaced funnels set flush in a countertop) have been developed. Suction tables are also available commercially.5 Regardless of what table is used, all exhaust air and solvent fumes should be vented into a fume hood. 9.2 ProcedureCover the suction table with polyethylene except for the small area to be used. Keep the area as small as possible for best suction. Lay thin blotter over the whole area exposed to suction. This method makes it easy to move the blotter as it becomes dirty without rearranging all the plastic mask. (Note: Never allow the object to be subject to suction while “bent” over the edge of a thick blotter. When the blotter covers the entire suction area, this can't happen.) A thin blotter is preferable because a thick blotter reduces the air flow needlessly. Lay the art object on the table. Where possible, place the side to which the tape was applied against the blotter. Any surface adhesive will then reach the blotter directly. When working near soluble media as in the case of the cartoon drawing, it may be necessary to keep the image face up so solvent can be applied precisely. In some cases soluble media may be placed face down if a polyester film (Mylar) mask is traced from the drawing, cut out, and laid over the verso to coincide with the design. Mask any exposed blotter with more polyethylene film. Where it was not possible to crepe away surface adhesive because of underlying friable media or soft-surfaced paper, dot on mild solvent and blot from the top while under suction. The mechanical action of blotting helps to remove sticky adhesive. Next, with either a brush, eye dropper, or air brush, apply solvent to remove adhesive that has penetrated the paper fibers. The size of the drop or the amount of solvent applied at once is important. The drop must be big enough to penetrate the paper and carry adhesive into the blotter below or the technique will not be effective. On the other hand, big drops spread unnecessarily and will form adhesive or paper-dye-laden tide marks far away from the original tape stain. For fine work, touch the paper surface lightly with the dropper while squeezing the bulb very gently. Release solvent only as it is absorbed by the paper. Move the dropper continuously to dry areas. Work several pieces of tape, or a sizable area, at once, because solvent must be added to dry paper. If the paper or blotter below is soaked with solvent, then additional applications will spread laterally in the object. Replace the underlying blotter as it becomes soiled. Be PATIENT; old adhesives that have increased in molecular weight take a while to soften and go into solution. This is normal behavior, so don't give up too soon! When all the adhesive seems to have been removed from a given area, test by brushing solvent evenly over the dry paper. Watch the spot as the solvent evaporates. Any remaining resin will trap the solvent and so dry more slowly than clean paper. This is a good way to check for adhesive tide lines that may not be visible in dry paper. If resin persists in spots, try more of the same solvent, or try both stronger or weaker solvents. Crusty patches can be rolled with tiny cotton swabs on the suction table. The slight mechanical agitation hastens break-up of the mass. Remove adhesive tide lines as they form. Brush or dot solvent directly on the line, then even the area by brushing outward from the center in a star pattern. Lightening the pressure on the brush as you move away from the adhesive puts an ever diminishing amount of solvent in the paper and ensures that no new tide lines will form. An air brush adjusted to deliver a very small, controllable, cone-shaped mist of solvent is ideal for this operation. When the adhesive has been removed from an area, a gray line may remain at the perimeter. This is surface dirt trapped by the sticky adhesive at the edges of the carrier. Dry cleaning with a vinyl eraser, off the suction table, may remove it. A golden stain may also remain after all adhesive has been removed if the tape carrier or adhesive When the tape crosses areas or lines of soluble media, remove the resin first in the largest open spaces. Gradually bring the solvent up to the edge of the soluble design. Adhesive cannot be removed from behind these areas and will remain in the art work. 10 CONCLUSIONIN THE RELATIVELY SHORT PERIOD since their invention in the 1920s, pressure-sensitive tapes have swept the world market. They are inexpensive, universally available, and convenient to use. These factors make them seem ideal for a variety of applications to archival materials and art on paper. However, time and experience have shown that pressure-sensitive tapes on paper are disfiguring, damaging, and difficult—sometimes impossible—to remove. Even the recent development of so-called archival tapes has not solved these problems from a conservator's viewpoint. The success rate for tape removal is much greater now than it was fifteen years ago. This progress can be attributed in part to increased understanding by conservators of the aging behavior of pressure-sensitive tape systems and the properties of organic solvents. It is also due to the availability of improved equipment and to the informed sharing of effective techniques. The three approaches to pressure-sensitive tape removal outlined above summarize the methods most frequently employed by the paper conservators in the Library of Congress Conservation Office. There are others that are equally effective. However, as long as pressure-sensitive tapes are available on the market, conservators can expect to be challenged by tape removal problems with elusive solutions. REFERENCESC. W.Bemmels, “Adhesive Tapes,” Handbook of Adhesives, Irving Skeist, ed., Huntington, New York, RobertE.Krieger Publishing Company, 1962. p. 584–592. The “Big Idea” that Changed the Habits of a Nation: “Scotch” Brand Pressure-Sensitive Tapes. St. Paul, 3M Minnesota Mining and Manufacturing Company, n. d.12 pp. Robert L.Feller and David B.Encke, “Stages in deterioration: The examples of rubber cement and transparent mending tape,” Science and Technology in the Service of Conservation: Preprints of the Contributions to the Washington Congress, 3–9 September 1982, London, IIC, 1982. p. 19–23. “Notes on the use of mending tapes on paper,” Bulletin of the American Group, International Institute for the Conservation of Historic and Artistic Works, Vol. 2, No. 1, October 1961. p. 13–15. At the time of writing, suction tables were commercially available from: Nascor Technical Services Incorporated, Box 706, Sag Harbor, L. I., New York, 11963; and 2) Process Materials Corporation, 301 Veterans Boulevard, Rutherford, N. J., 07070 or Fine Arts Fabricators, 4230 Howard Avenue, Kensington, Md., 20895.
 Section Index Section Index |