A CAUTIONARY NOTE ON THE PRESENCE OF SILVER CYANIDE ON MUSEUM OBJECTSDonna Strahan
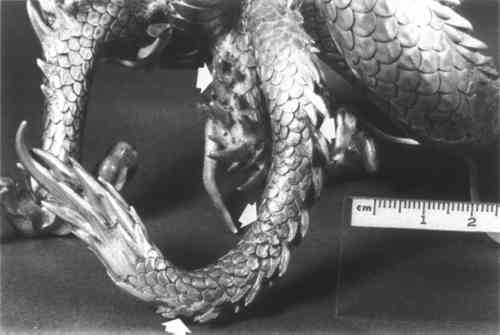
ABSTRACT—A 19th century Japanese silver dragon was discovered to have a silver cyanide corrosion product scattered over its surface. The corrosion product appears as a gray to black, wart-shaped powdery substance on the silver surface. Due to the extremely poisonous nature of cyanide salts and gases caution should be taken when handling and treating suspected silver objects. A MEIJI PERIOD (late 19th century) silver Japanese dragon with gilt claws and tongue was brought from dark storage into the conservation laboratory at the Walters Art Gallery due to the presence of surface corrosion. Small patches of whitish-gray corrosion were scattered unevenly over the cast silver surface. After roughly a month in the laboratory it was noted that the whitish-gray deposit was slowly converting to a charcoal black color. A small sample was sent for analysis∗∗ and identified as silver cyanide
∗∗X-ray diffraction analysis was generously performed by Paul Jett of the Technical Laboratory of the Freer Gallery of Art and also by Martha Goodway of the Conservation Analytical Laboratory, the Smithsonian Institution.
To determine why the product developed on the object, research was carried out on its method of manufacture and past conservation treatments. After reviewing the registrar's records it was discovered that the dragon had been sent to a silver company in the early 1960's for cleaning. Commercial silver companies commonly clean objects by dipping them in a dilute solution of cyanide which is prepared from sodium or potassium cyanide. Normally the objects are thoroughly rinsed after the bath and then hand buffed. Incomplete rinsing can leave behind a residue of the cyanide solution, which can react with the silver object and form silver cyanide. This industrial cyanide cleaning method has been used for decades and some museums may have had their historical silver commercially cleaned sometime in the past. Therefore, for health reasons close examination should be given to all silver objects before any conservation treatments commence, particularly if the treatments involve acids. Silver cyanide (AgCN) is an extremely poisonous grayish-white odorless powder which darkens when exposed to light. Although it is not readily absorbed through the skin, it is deadly by inhalation or ingestion. Acids readily react to break down the complexed metal salt, producing highly poisonous hydrogen cyanide gas which has a characteristic odor of bitter almonds. Any treatment involving acid in contact with silver cyanide could prove fatal. Therefore, any whitish-gray or black powdery wartshaped product found on historical silver objects should be handled with caution before conservation treatment is begun. If the product is suspicious all treatments should be carried out wearing gloves in a fume hood, and any corrosion dust should be disposed of properly.
 Section Index Section Index |