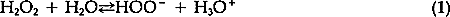THE BLISTERING OF PAPER DURING HYDROGEN PEROXIDE BLEACHINGDaniel Clement
ABSTRACT—An experiment is described which relates the amount of damage to certain degraded papers caused by the formation of gas-incurred delaminations during aqueous hydrogen peroxide bleaching to variations in treatment conditions. While some treatment modifications decreased the severity of the blistering, none prevented the problem entirely. 1 INTRODUCTIONMANY PAPER CONSERVATORS USE hydrogen peroxide to bleach paper which has become discolored or stained. This bleach offers advantages over some of the other common bleaches. It decomposes into oxygen and water so that there is less chance that dangerous chemicals will be left in the paper; for example, it does not contain chlorine and so cannot leave chlorine residues. According to Burgess and Hanlan, hydrogen peroxide tends to degrade cellulose less than other common bleaches.1 While some conservators have noted problems of color reversion, Burgess reports results which indicate that paper bleached with hydrogen peroxide is less prone to color reversion than paper bleached with other common bleaches.2 Also, information presented in a TAPPI publication suggests that hydrogen peroxide improves the color stability of both bleached chemical paper pulps and bleached mechanical paper pulps.3 One problem that conservators sometimes encounter with aqueous hydrogen peroxide bleaching, however, is that blisters may form in the paper during bleaching. These blisters result when gas is produced and captured within the paper structure, causing small pockets. These gas-produced delaminations are visible because they stand in relief from the plane formed by the sheet of paper. While it is true that during actual treatments the bleaching can be terminated when the blistering starts to occur and the blisters can usually be brought back into plane as the paper dries, such mechanical disruption of the paper certainly is to be avoided. The active bleaching species4 associated with hydrogen peroxide is the perhydroxyl anion, HOO−. The reversible equilibrium which determines the concentration of this anion is described by
The irreversible decomposition of hydrogen peroxide, on the other hand, is described by
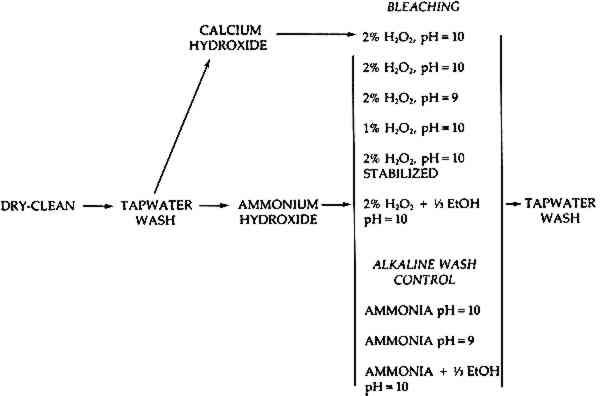
The present study reports results obtained in an experimental investigation into the extent of blistering during the bleaching of selected old papers when treated in a number of different hydrogen peroxide baths. The objective was to see which bleaching conditions and pretreatment procedures produced the least blistering for a given amount of bleaching. 2 EXPERIMENTAL PROCEDURESeven degraded expendable nineteenth and early twentieth century lithographs were chosen as subjects for the experiment. These prints were cut into small pieces and the pieces evenly distributed into several groups which would each receive a different treatment. In this way, a number of treatments could be performed on parts of the same print and the variations in the amount of blistering and bleaching could be noted. Variations in bleaching procedures included changes in pH or concentration, variation in the kind of alkaline wash pretreatment, and the addition of ethanol or stabilizers to the bleaching bath. The amount of blistering and bleaching was measured after each treatment. The expendable artifacts used are described briefly in Table 1. These prints were chosen because in a preliminary screening they were all shown to be susceptible to the formation of blisters during bleaching.7 Old prints were chosen in preference to other Table 1. Description of the Papers Used The purpose of the investigation was not to determine what kinds of paper were more likely to blister, but to see whether the problem of blistering could be abated by varying bleaching conditions. The treatment procedures are described below and they are also outlined by the flow chart in Figure 1.
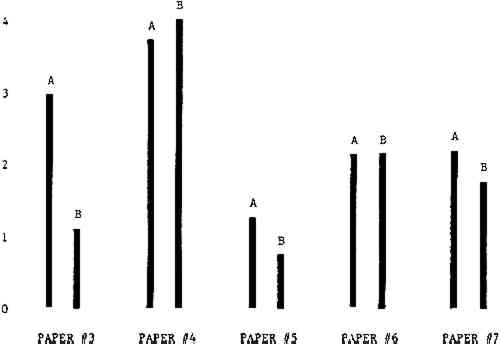
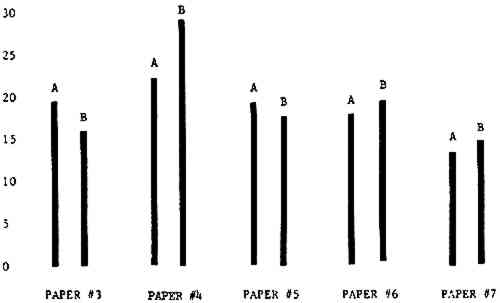
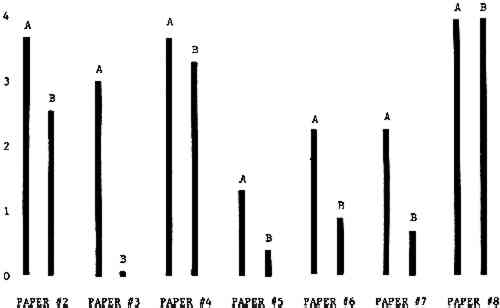
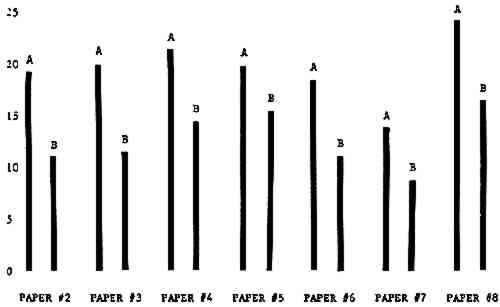
3 RESULTSData obtained in the experiment are summarized in the form of bar graphs (Figures 4–8). Each figure shows, for a number of different papers, the amount of bleaching and blistering that took place under the standard procedure as opposed to that which occurred under a modified procedure. The results obtained with each paper under the standard conditions are always represented by the bar (marked A) at the left in each pair of bars. The bar at the right (marked B) in each pair is the experimental variation which is being compared to the standard pH 10—2% procedure.
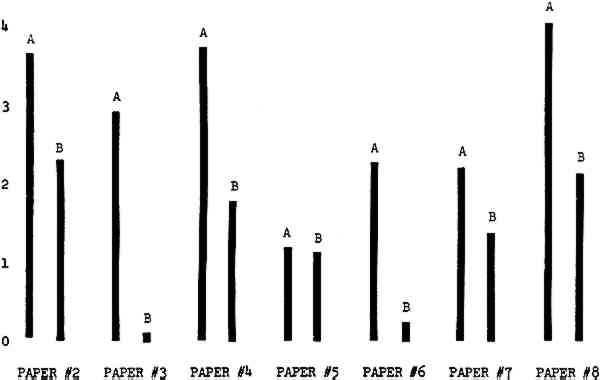
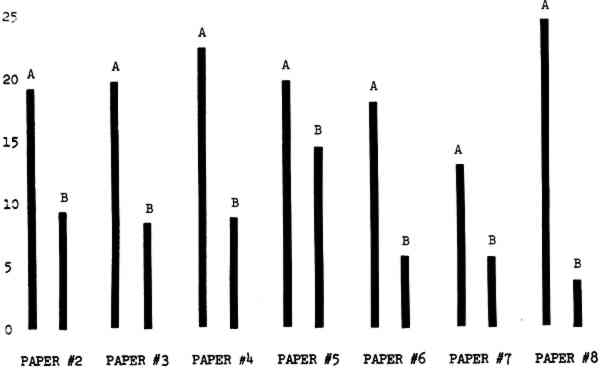
3.1 A. Calcium Hydroxide vs. Ammonia Pre-treatment
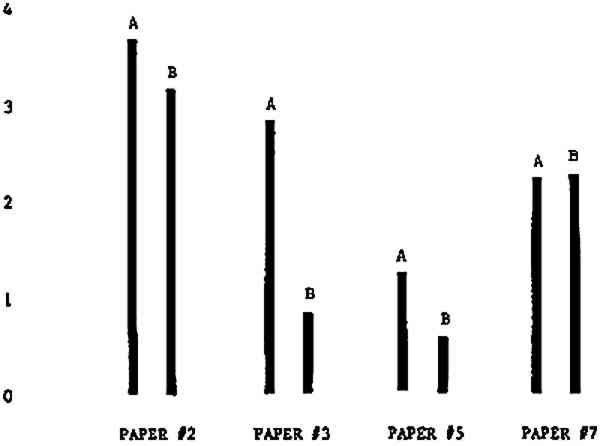
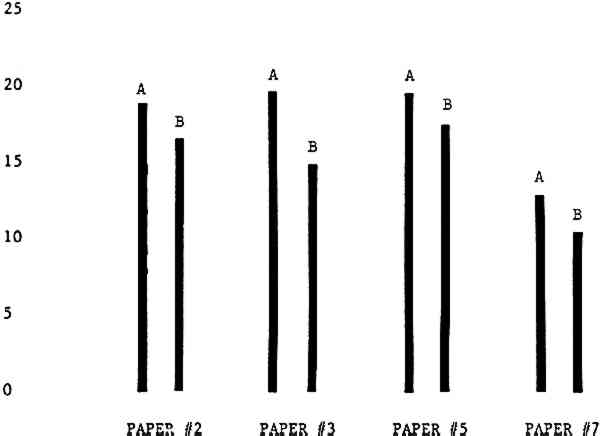
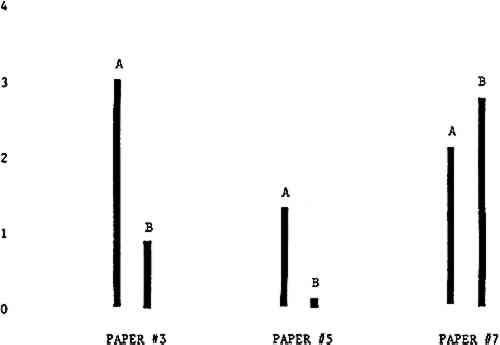
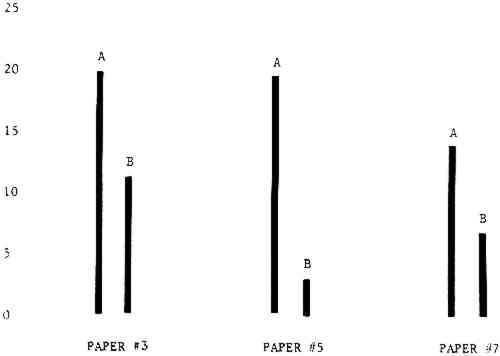
Figure 4A shows the amount of blistering that occured during bleaching and Figure 4B shows the increase in brightness. The amount of blistering was reduced in most, but not all cases. The amount of bleaching was slightly reduced in all cases, possibly because more discoloration was removed in pre-treatment due to the higher pH of the calcium hydroxide bath. 3.2 B. pH 9 vs. pH 10Figures 5A and 5B show that lowering the pH reduced both the amount of blistering and the amount of bleaching. This is consistent with the fact that the concentration of the active bleaching species and the rate of hydrogen peroxide decomposition are both less at lower pH. It is notable that the various papers reacted quite differently to the same variation in bleaching conditions. Blistering was reduced significantly with papers #3 and #4 without equally great reductions in bleaching. Little reduction in blistering occurred with paper #5. Although blistering was reduced significantly with paper #8, the reduction in bleaching was considered unacceptably great. The papers were obviously quite different in their reactions to changes in bleaching conditions, but the differences would probably not have been predictable by the paper conservator prior to bleaching. 3.3 C. 1% vs. 2% ConcentrationThe results given in Figures 6A and 6b show how three different papers reacted when the hydrogen peroxide concentration was lowered to 1% from the standard of 2%. With papers #3 and #5 blistering was substantially reduced, but not with paper #7. The amount of bleaching, however, was reduced in all cases. It is apparent that the amount of oxygen produced can be very substantial even with lower concentrations of hydrogen peroxide. The decomposition of even a small amount of hydrogen peroxide can produce a surprisingly large volume of oxygen. (The decomposition of 1 ml of a 1% solution can produce approximately 5 ml of gas.) 3.4 D. Stabilized vs. UnstabilizedFigures 7a and 7B show that with paper #3 the amount of blistering was greatly reduced while the amount of bleaching remained about the same. Unfortunately, such encouraging results were not produced with the other four papers. These results seem consistent with a statement by Schumb, Satterfield and Wentworth: “… it appears reasonably certain that in the presence of more than minute proportions of catalytically active contaminants it is impossible to restrain the decomposition of hydrogen peroxide by the addition of stabilizers …”15 It seems likely that the amount of impurities present in papers like these may be beyond the limit within which stabilizers are effective. It is worth noting that each time this bleach bath was prepared a certain amount of the white chemicals remained undissolved. This material clouded the solution and partially obstructed the view of the artwork. This phenomenon might restrict the usefulness of the bath for conservation procedures since the paper must be observed carefully during the bleaching process so that if blistering starts to occur the bleaching can be terminated. Also, the insoluble material might become trapped in the interstices of the paper. 3.5 E. Addition of EthanolIn the final variation of treatment, ethanol was added to the bleaching bath. It was hoped that in the presence of ethanol the paper might remain stronger because of the smaller percentage of water. Ethanol reduces the surface tension, thus increasing the possibility that the gas produced could escape from the paper structure without damaging the paper. Oxygen is also much more soluble in ethanol than in water, a fact which might reduce the extent of bubble formation. Figure 8A shows that the addition of ethanol reduced the amount of blistering in most but not all cases. Again, the various papers reacted to the same change in procedure quite differently. The addition of ethanol practically eliminated the blistering with paper #3, but did not substantially reduce the blistering with papers #4 and #8. Blistering was quite reduced also with papers #5, #6 and #7. The amount of bleaching was reduced in all cases. 3.6 F. Marginal PiecesAs mentioned earlier, note was made of which pieces came from the margins of the prints and these pieces were distributed among the different bleaching baths. Indeed, it was found that the amount of blistering on these pieces was greater than average by about half a point in the 0 to 4 scale used to describe damage. 3.7 G. Alkaline Wash ControlsThe amount of brightening due to alkalinity alone was always very small compared to the effect due to bleaching.16 3.8 H. pH Changes During BleachingThe alkalinity of the bleaching baths never changed by more than 0.15 pH unit during treatment, and generally the changes were much less than this. These fluctuations were judged not to be significant. 4 CONCLUSIONSSeveral conclusions about the blistering of paper from aqueous hydrogen peroxide bleaching can be drawn:
5 SUMMARYTHE VARIATIONS IN bleach bath composition and paper pretreatment considered in this experiment reduced the tendency of the paper to blister in certain cases. While the use of a less alkaline solution and the addition of ethanol showed promise, no test procedure eliminated the problem of blistering. No “miracle cure” was discovered for this problem, and the conservator should always be aware that blistering may occur even if precautions are taken. Certainly, further research is warranted. ACKNOWLEDGEMENTSTHIS RESEARCH WAS performed while the author was a student at the Cooperstown Graduate Program. The author would like to thank the faculty, especially Dr. Christopher Tahk and Ms. Cathleen Baker, and his fellow students for their help and guidance. REFERENCESBurgess, H. and Hanlan, J., “The Degradation of Cellulose by Bleaching,” Journal of the International Institute for Conservation—Canadian Group, Vol. 4, No. 2 (1979): pp. 15–22. Burgess, H., “The Colour Reversion of Paper After Bleaching,” preprints of the Institute of Paper Conservation Cambridge Conference, 1980: pp. 171–183. Rapson, W. H., ed., The Bleaching of Pulp, Technical Association of the Pulp and Paper Industry, New York (1963): p. 301.
The dissociation constant of hydrogen peroxide in an aqueous solution at 25�C is Schumb, W.; Satterfield, C.; Wentworth, R., Hydrogen Peroxide, Rheinhold Publishing Corp., New York (1955): Chapter 8. When the gas which formed within the blisters was exposed to a small glowing splint of wood, the wood immediately underwent very rapid combustion, indicating the presence of oxygen. To see whether a particular print was prone to blistering during hydrogen peroxide bleaching, a small section of the sheet was placed in a 2% solution of peroxide in water (pH = 10). The papers which blistered were chosen for the experiment. The recommendation for calcium hydroxide (or magnesium bicarbonate) pretreatment is found in: Hey, M., “Paper Bleaching: Its Simple Chemistry and Working Procedures,” The Paper Conservator, Vol. 2 (1977) P. 20. The use of a 50% saturated solution of calcium hydroxide is suggested in:Hey, M., “The Washing and Aqueous Deacidification of Paper,” The Paper Conservator, Vol. 4 (1979): p. 70. In the author's opinion, this solution is too alkaline for routine treatment of works of art. The water was deionized with a Barnstead Combination Cartridge. All chemicals were Fisher Scientific Reagent Grade. A pH of 10 was chosen because it allowed comparison with Ms. Burgess' stabilized bleaching bath, where a pH of 10 is specified. (See #1 of #2 above.) Burgess, H., “The Colour Reversion of Paper After Bleaching,” (op. cit.): p. 172. In this case, the resulting concentration of hydrogen peroxide in water and ethanol was less than 2%. It was decided for the purposes of the experiment not to consider the alcohol as part of the solvent since in preliminary tests it seemed to be a relatively inert component in the peroxide-water-alcohol-ammonia bleaching bath. Diffuse reflectance measurements were taken using a Pye Unicam SP8-100 spectrophotometer equipted with a diffuse reflectance accessory (an integrating sphere type conforming to C.I.E. recommendations) and a barium sulfate white reflectance standard. The reflectance measurements were the same whether the supporting layer behind each sample was white or black. The wavelength of 416 nm was chosen because it allowed comparison with work done by Ms. Burgess. Also, when some full spectrum readings of paper and after bleaching were studied, it was observed that the greatest changes in reflectance did occur in the blue part of the spectrum near 416 nm. Schumb, W.; Satterfield, C.; Wentworth, R., Hydrogen Peroxide (op. cit.): p. 535. In the interest of brevity, and because the amount of brightening due to alkalinity was small, the data obtained with these controls are not considered in this paper. Complete data may be obtained from the author. P.Holladay and R.Solari mention in The Bleaching of Pulp (op. cit.: p. 191) that catalase is formed by the metabolism of bacteria which are found in some paper pulps. The production of this enzyme is of concern to papermakers because it accelerates hydrogen peroxide decomposition and therefore reduces bleaching efficiency. It would be interesting to see whether this enzyme plays a role in the more vigorous decomposition of hydrogen peroxide noted in degraded paper.
 Section Index Section Index |