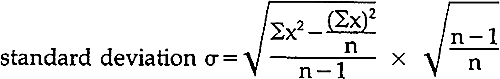EFFECTS OF WASH WATER QUALITY ON THE PHYSICAL PROPERTIES OF THREE PAPERSJ. Nelson, A. King, N. Indictor, & D. Cabelli
ABSTRACT—The effects of dry oven accelerated aging on samples of three kinds of paper (Whatman Chromatography #1, Arches Cover Infinity, and Aquabee Newsprint Rough 887-R), washed in three qualities of water (New York City tap water, deionized, and distilled), were observed. Changes in folding endurance, tensile strength, reflectance, color, and pH are reported. Results support findings reported by other researchers that greater purity of wash water is not necessarily beneficial to paper. However, the findings presented here suggest that the acidity of the wash water and the composition of the paper are also factors influencing the effects of the washing treatments. 1 INTRODUCTIONTHE CHARACTERISTIC EFFECTS of water on such properties as strength, rigidity, elasticity, and flexibility of papers and paper-based products have long been recognized in industry as dependent on constituent fibers, fillers, and sizing.1 Only relatively recently, however, has the effect on paper of water as a chemical reagent come under the scrutiny of conservators. In a recent publication, Tang and Jones report results of a study aimed at establishing guidelines for acceptable water quality for paper conservation treatments.2 To document the effects of water washing on paper properties, they treated two types of paper with five types of water, varying both water purity and temperature. There was a correlation between water purity and pH, i.e., the extra pure waters were acidic, while the less pure waters were alkaline. The paper samples were relatively new newsprint and Foldur Kraft papers, both acid papers considered to have little permanence. Tang and Jones found that highly purified water such as distilled or deionized water seemed to strip paper of beneficial constituents such as calcium, and they suggest that this might be why these samples showed a decrease in physical strength after artificial aging. Of great interest was the finding that the Washington DC tap water seemed to have a beneficial effect on the tested papers. In addition to calcium, this water was reported to contain significant amounts of the destructive agents chlorine, copper, and iron. The present study had two objectives: first, to try to reproduce the observations of Tang and Jones, that different qualities of water have different effects on the strength of papers washed in them; second, to see if the effects observed on relatively impermanent papers would be apparent on more permanent papers as well. 2 EXPERIMENTAL PROCEDURE2.1 MaterialsTHREE DIFFERENT TYPES of paper were selected, based on an estimate of permanence. Permanence can be defined for the purposes of this study as the expected ability of the paper to retain certain properties (i.e., those properties tested, as described below) during the various experimental treatments. The papers selected for testing were:
Three waters were selected to give a range of purity:
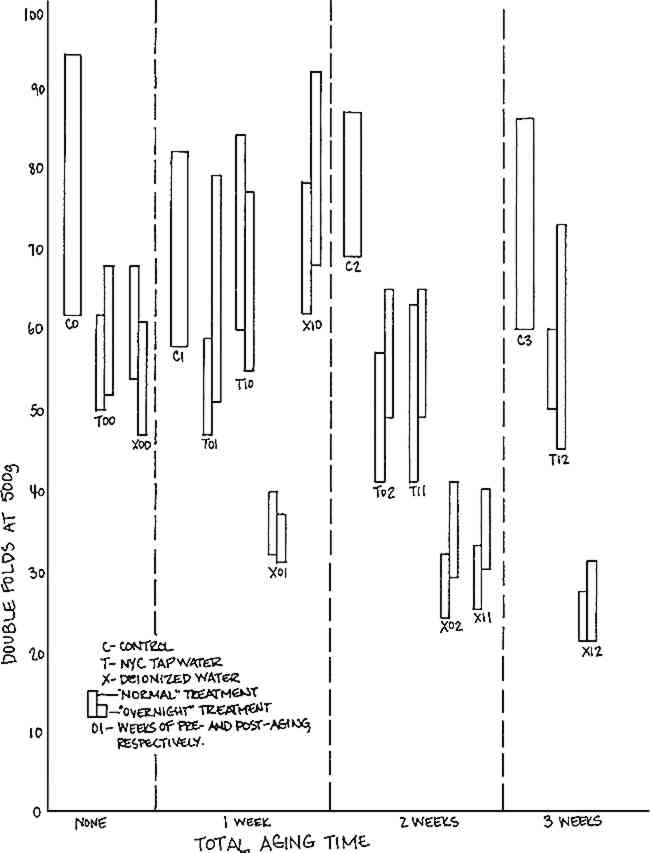
All three types of water were tested for free and total chlorine content and for hardness as calcium carbonate.11 Both the deionized and distilled waters showed no perceptible amounts of chlorine or calcium carbonate, while the tap water did contain significant amounts of both contaminants (0.1mg/1 chlorine, and 102.6mg/1 calcium carbonate). Each of the waters was tested for pH (see Table I). Both the tap and deionized waters had values that varied from day to day, but the pH of the individual samples of the water remained stable for periods of two to three hours. The pH of the distilled Table I. pH of Wash Waters 2.2 Methods of TreatmentTen samples of each paper were subjected to each of the various combinations of washing and aging procedures. To minimize differences in handling, the ten samples for each treatment were sewn to a strip of Whatman Chromatography #1 paper, leaving a gap of approximately 3/8″ between the samples to allow free circulation of the water on all surfaces.13 Nylon net was used as a washing support. Preliminary tests of the effect of the net when used as a washing support for Whatman paper samples revealed an increase in pH and in the folding endurance of the paper samples after washing. The net itself was considerably more flexible after the treatment. This strengthening effect on the paper was attributed to the action of a water-soluble sizing on the net. The infrared spectrum14 of a sample of the sizing showed a strong resemblance to polyvinyl alcohol, which has been found to cause a large increase in the folding endurance of paper.15 The net was considered suitable for use as a washing support when further preliminary washing did not result in a change in its flexibility or in the pH of the wash water. This was achieved by bathing the net for one half-hour in hot tap water. Folders of the net were made by folding in half sheets approximately 14″�14″, and sewing with white cotton thread along the fold. Before each treatment, the prepared net folders were soaked for about 15 minutes in the type of water to be used in the treatment, then drained on clean blotters, and allowed to air dry. The paper samples were placed in the net folders with the support strip of Whatman paper adjacent to the folded seam. This system provided consistency in handling, as all ten samples could be picked up at the same time, being held through the two layers of net by the corners of the support strip. The washing trays were constructed of Mylar, a chemically inert film of polyethylene terephthalate.16 Just before each treatment, the washing trays were rinsed with tap water, then with deionized water for the deionized and distilled water treatments, and thoroughly drained. Each washing tray held two liters of water, maintained at 21�-24�C for the tap water and deionized water, and at 18�C for the distilled water. (Temperature was monitored with recording hydrothermographs. Nonuniformity in the treatment temperature resulted from changes in outside temperature for which the heating system could not adequately compensate.) The washing treatment consisted of submersion of each folder of samples in three successive baths. “Normal” wash time was one half-hour (ten minutes per bath); “overnight” wash time was 16� hours (ten minutes each in the first two baths, and sixteen hours ten minutes in the last bath). Repeated immersion in still water baths was chosen over a single immersion in running water primarily for the sake of convenience. The solvation of material during washing has been shown to be dependent more on its diffusion into the wash water than on the amount of flow of the water, a bath of still water being adequate for washing if the water-paper system is allowed to reach equilibrium.17 We felt that if equilibrium were not reached with two liters of water and ten minutes wash time per bath, then a) these conditions at least approximate common practice and are thus reasonable, and b) this would show up in a difference in effect between the “normal” Samples were aged in a Precision Scientific Company Model 17 circulating dry oven at 100�3�C. The samples were hung vertically from a rack, very loosely packed to ensure free circulation of air around all surfaces of each sample.18 To separate the effects of washing from effects of dry oven aging, unwashed control samples of each type of paper were given aging treatments identical to those of the experimental samples. Whatman paper samples were subjected to a range of aging times: 0 or 1 week before washing; and 0, 1, or 2 weeks after washing; referred to as pre- and post-aging respectively. Arches paper samples were post-aged only, for 0, 1, and 2 weeks. Newsprint samples were post-aged only, for 0, 3, and 7 days. Dry oven aging was chosen as a variable to determine whether effects of washing not immediately apparent would be manifested with the increase in temperature. It should be noted that this was not intended to approximate natural aging.19 The reactions that occur during dry oven aging, affecting folding endurance, brightness, and pH, proved to be sensitive to water washing, and as such are good indicators of differences in the effects of the various waters. 2.3 Methods of TestingFolding endurance was measured on a Tinius-Olsen Model No. 2 machine,20 with a dead weight of 250g on a #2 spring for Newsprint, 500g on a #2 spring for Whatman, and 750g on a #3 spring for Arches samples. Results are reported as the average of ten measurements with standard deviation.21 Tensile strength measurements were made on an Instron Table Model TM-M 1101 Universal Tester,22 with crosshead speed of 0.5cm/min, and load scale of 10kg for Whatman and Newsprint, and 20kg for Arches samples. Determination of tensile strength was made on portions of the samples not previously subjected to tension during folding endurance testing. Results are reported as the average of three to ten measurements with standard deviation. Color was subjectively measured by comparing samples to each other and to Munsell Color Company glossy surface sample chips23 under illumination from the Macbeth Spectral Light Machine at the setting which revealed the most differences in color: “daylight” setting for Whatman and Arches samples, and “minus red” for Newsprint samples. Reflectance was measured as percent-transmittance at 457nm on a Bausch & Lomb Spectronic 20 Colorimeter-Spectrophotometer.24 For each type of paper, 100% transmittance was defined by the reflectance of the unaged, unwashed control samples. Results are reported as the average of three measurements with standard deviation. Surface pH was measured on a Beckman Expandomatic SS-2 pH meter25 with an Ingold silver chloride reference glass electrode.26 Readings were taken on samples laid out on a sheet of Mylar over a sponge pad, rinsed with distilled water and dried between readings. The electrode was also rinsed with distilled water and dried between readings. A value for surface pH was obtained as follows: one drop of nitrogenstabilized distilled water was placed on the sample. Whatman paper, which wet easily, was tested by placing the electrode on the wetted surface and immediately commencing the reading. With Arches and Newsprint samples, the drop of distilled water sat on the surface and was moved in a small arc with the electrode to enhance wetting before the reading was taken. A value for each reading was obtained when the 3 RESULTS3.1 Whatman Chromatography #1THE RESULTS OF TESTING CONTROLS and treated Whatman paper samples with various combinations of pre- and post-aging are summarized in Table II. Due to limitations on the size of the sample population, Whatman samples were washed in New York City tap water and deionized water only. (It proved to be an unfortunate choice to investigate differences in pre- and post-aging at the expense of the distilled water treatment, which was postulated to have an effect similar to that of deionized water. That pre-aging would have no effect and that there would be differences between the effects of distilled and deionized waters could not have been known before testing was completed and the data analyzed.) All samples were uniformly bright and exhibited no change in color, and negligible change in tensile strength, regardless of treatment. Changes were observed in folding endurance and pH. Table II. Effects of Wash Waters on Physical Properties of Whatman Chromatography #1 Paper Changes in folding endurance are represented in Figure 1. Values for the controls remain constant regardless of aging, indicating that oven aging alone had no effect. Values for the samples washed in tap water overlap the control values substantially; thus, no clear effect can be attributed to tap water washing. There is evidence of mechanical weakening from washing in deionized water with post-aging: all eight sample sets receiving this combination of treatment suffered approximately 50% loss of folding endurance. The four sample sets washed in deionized water without postaging had folding endurance comparable to the control and tap water washed samples. Pre-aging of samples made no perceptible difference in the response to washing and post-aging.
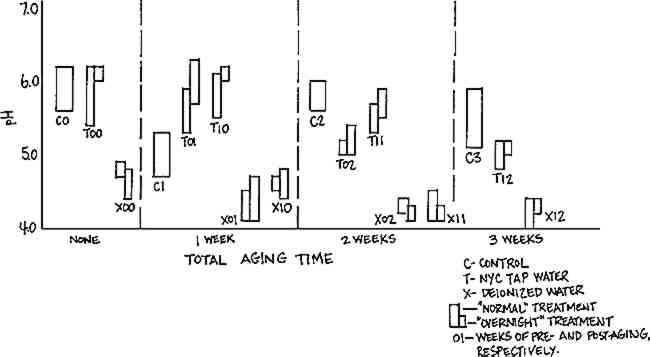
Results of pH testing, illustrated in Figure 2, indicate a drop in pH of all samples washed in deionized water, suggesting that an alkaline component of the Whatman paper is removed in washing with this purified water. This strongly suggests that the cause of the changes seen in folding endurance is associated with the washing, although only activated by the post-aging procedure.
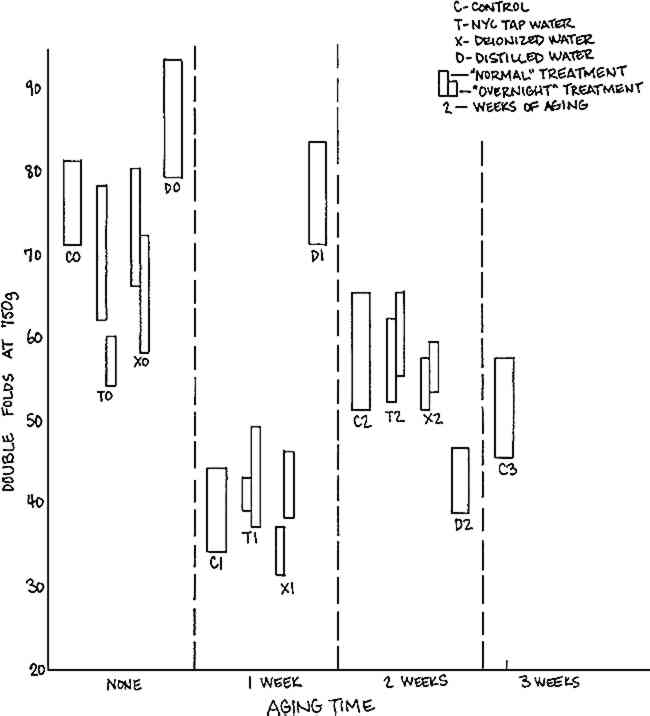
3.2 Arches Cover InfinityThe results of testing controls and treated Arches Cover Infinity samples with various times of post-aging are summarized in Table III. There was no change in color, a slight decrease in brightness with aging regardless of washing treatment, and inconclusive changes in tensile strength. Again, changes were seen in folding endurance and pH. Table III. Effects of Wash Waters on Physical Properties of Arches Cover Infinity Paper Results of folding endurance are illustrated in Figure 3. There is a general downward trend with aging, with a significant drop in folding endurance for some samples aged one week. This drop and subsequent rise could be the result of a number of things, for example, possible cross-linking of a sizing agent which could compensate for deterioration due to aging.28
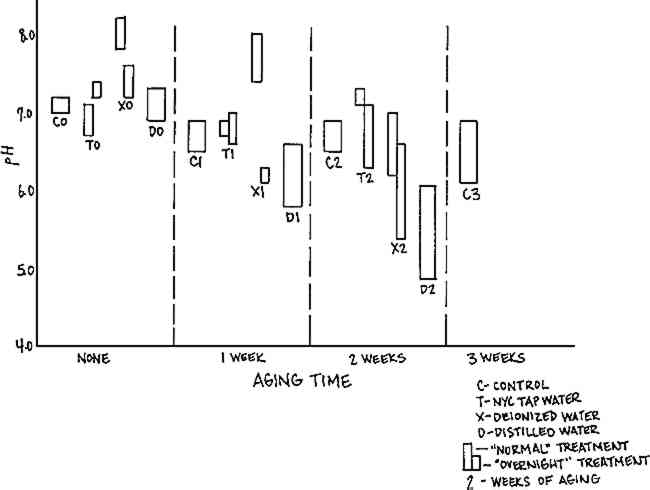
The values for folding endurance after distilled water washing do not follow the same pattern as those for the other treatments: there is a rise in folding endurance with one week oven aging which is difficult to explain. It is possible that the nitrogen in the water, or the lack of oxygen, had a relatively protective effect which was not dominant after more than one week oven aging, so that after two weeks aging, the samples Effects of washing on the pH of the Arches samples, illustrated in Figure 4, are not correlated with the effects on folding endurance. The pH is stable on aging regardless of treatment except for the samples washed for one half hour with deionized water and post-aged zero or one week. These show a slight increase in pH compared to the other samples. An explanation is suggested by the correlation of the increase in pH with the wash time. Short-term wetting with the deionized water could have resulted in an initially higher surface pH due to a concentration at the surface of an alkaline component of the paper, which would be either re-distributed in the paper or diffused into the wash water with the longer wash time.
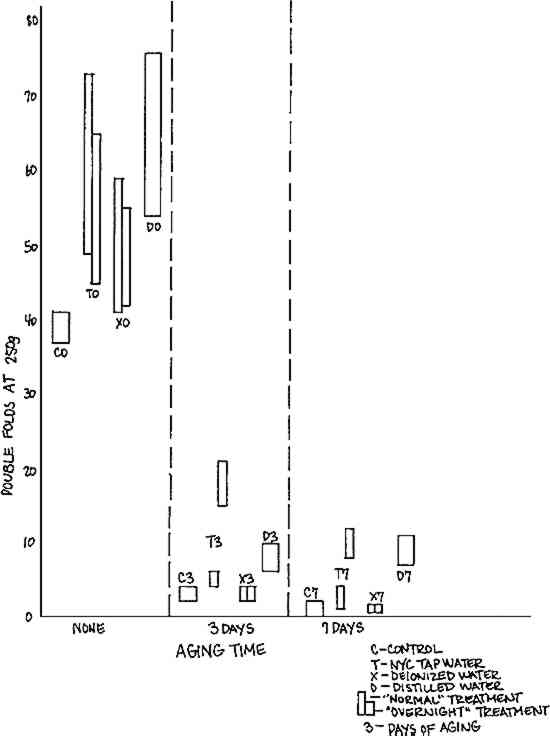
3.3 Aquabee Newsprint Rough 887-RThe results of testing controls and treated samples of Newsprint with various times of post-aging are summarized in Table IV. There were inconclusive changes in tensile strength. Changes in color were dramatic and could be correlated with changes in reflectance. The trends, which were towards increased yellowing and decreased reflectance with aging of controls, were exacerbated by washing in deionized water, greatly inhibited by washing in tap water, and somewhat inhibited by washing in distilled water. Table IV. Effects of Wash Waters on Physical Properties of Aquabee Newsprint Rough 887-R Paper The precipitous decrease in folding endurance with aging is illustrated in Figure 5. All washing treatments without aging have a strengthening effect, which persists on aging only for the tap water and distilled water treatments. The protective effect of tap water washing on post-aged samples is apparent after the “normal” half-hour wash time, but is greatly increased with the increase in wash time to the “overnight”
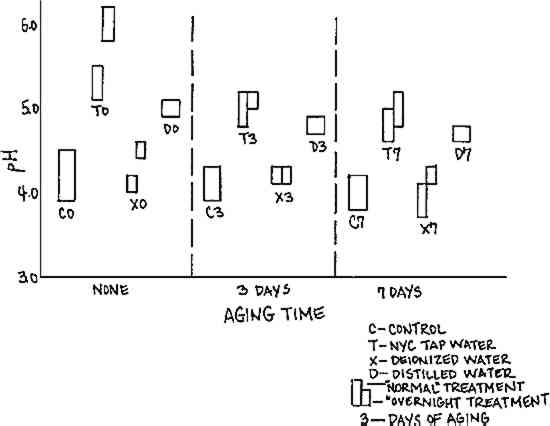
Results of pH testing, illustrated in Figure 6, show a corresponding increase in pH for all samples washed in tap and distilled water. These results suggest the deposition in the paper during the wash treatment of some protective alkaline component of the tap and distilled water (which had pH 7.1-7.8), or alternatively, the removal from the paper during the wash treatment of some deleterious acidic component of the paper.
4 DISCUSSIONDATA OBSERVED INDICATE that the three waters used had different effects on the papers tested. Within the sensitivity of the water testing kits used in this study, no difference in composition is perceptible between the deionized and distilled waters. However, the short-channel single distillation procedure used would not be expected to purify the water as well as the deionization procedure; the distilled water would be expected to contain more inorganic contaminants. This is suggested to be the case by the slightly alkaline pH of the nitrogen-stabilized distilled water, and by the effect of the distilled water wash on the newsprint samples. As noted previously, in the Tang and Jones study there was a correlation between the purity and pH of the wash water and its effect on the aging characteristics of the samples, with samples washed in the more pure, more acidic waters performing less well in accelerated aging tests than both unwashed controls and samples washed in more alkaline waters. Tang and Jones suggest this results from the loss of calcium due to the aggressiveness or increased solvent power of the ion-poor pure waters,29 while others have suggested that the acidity of the purified waters used in that study might have been a factor.30 In the present study, the pH of the deionized water and of the New York City tap water were comparable, and the more pure deionized water wash was deleterious to some of the papers, while the less pure New York City tap water wash was not, supporting the interpretation of Tang and Jones. On the other hand, the purity of the 5 CONCLUSIONSTHE EFFECTS OF WASHING PAPERS of varying permanence in waters of varying purity, as observed under the described conditions, are not clear cut. A number of issues would bear further study. However, it is clear from this preliminary work that the different papers do not respond uniformly to given conditions, and that each paper responds variously to differing conditions. Thus, findings obtained by evaluating any one kind of paper do not appear to be universally applicable. However, certain observations may be made on the basis of the data presented here. New York City tap water was not shown to be deleterious under any conditions. However, as noted in the Tang and Jones study,31 tap water quality is subject to great variability, and cannot be recommended without reservation. Likewise, the nitrogen-stabilized distilled water was shown to be deleterious. But since the nature of its inorganic contaminants is dependent on the composition of the tap water from which it is distilled, neither can it be recommended. The deionized water was shown to be deleterious to the pH of the two papers expected to be the least permanent and moderately permanent, Aquabee Newsprint Rough 887-R and Whatman Chromatography #1, respectively, and to their folding The findings suggest: 1) that highly purified wash waters are capable of removing a substance from some of the papers treated, while the alkaline wash waters are capable of depositing a substance in some of the papers treated, in this study both substances being alkaline salts; 2) that the substance is beneficial, i.e., its presence causes the paper to retain properties that are otherwise altered by dry oven aging; and 3) that the more of the substance there is, whether deposited during manufacturing processes or by the washing treatment, the greater is its beneficial effect. REFERENCESASTM Special Technical Publication No. 60-B. Paper and Paperboard Characteristics, Nomenclature, and Significance of Tests, 3rd Edition. Part II, p. 11–14 discusses “The Action of Water on Paper and Its Significance.” Lucia C.Tang and Norvell M.M.Jones, “The Effects of Wash Water Quality on the Aging Characteristics of Paper,” Journal of the American Institute for Conservation, Vol. 18 (Spring 1979): 61–81. Manufactured by Whatman Inc., Whatman Paper Division, 9 Bridewell Place, Clifton, NJ 07014. Laboratory Catalog 1979 Paper Products, Publication 800, Whatman Inc. (1979), p. 24; and Paper Chromatography Laboratory Guide, Bulletin No. 201, Whatman Inc. (1977), p. 14 and p. 17. The authors thank Ellen Pearlstein for sharing her research on Whatman paper. Manufactured by Arjomari, S.A., 3 Rue du Pont du Lodi, Paris 6 France, represented in the United States by Special Papers, RFD 2, West Reading, CT. The distributors of this paper are authorized to state only that it is 100% rag, buffered with 3% calcium carbonate, and has a synthetic size. (Ms. Vera Freeman, Manager, Art Paper Dept., Andrews/Nelson/Whitehead, 31-10 47th Avenue, Long Island City, NY.) The angle between the paper surface and the sides of a drop of water placed on it is acute, as described in TAPPI T-458 os-70, indicating that the paper is hard-sized. Microscopic examination of several samples failed to reveal fibers other than cotton. Machine direction of the papers used in this study was determined by flexing as described in TAPPI T-409 os-61. Manufactured by Bee Paper Co., 100 8th Street, Passaic, NJ. The specifications for this sheet are “novel news; 321b/500 (24�36) sheets; rough, toothy finish; well-sized; clean.” Its composition is approximately 80% groundwood, with the remaining 20% being chemical woodpulp, from either a sulfite or sulfate process, at the mill's discretion. The nature of the size is likewise at the mill's discretion. (Mrs. Bee, President, Bee Paper Co.) Tests for alum and rosin gave positive results, indicating the presence of alum-rosin size. (W.J. Barrow Research Laboratory, Inc. Permanence/Durability of the Book—VI, Spot Testing for Unstable Modern Book and Record Papers, p. 12–13.) Manufactured by Barnstead, Division of Sybron Corp., 225 Rivermoor Street, Boston, MA 02132.
The free and total chlorine test kit, Hach Model CN-70, and the hardness as calcium carbonate test kit, Hach Model HA-71A, were obtained from Hach Co., PO Box 389, Loveland, CO 80537. The use of nitrogen gas for the stabilization of distilled water is suggested in TAPPI T-509 su-68. This technique was suggested by Debbie Trupin. The authors thank Mr. Tony Frantz, of the Objects Conservation Dept. of the Metropolitan Museum of Art, for the use of the infrared spectrophotometer in his department. M.G.Blank, “The Effect of Polymer Additives on the Strength of Paper of Different Compositions,” Restaurator, Vol. 2 (1978): 155–162, p. 161. For characterization of polyester films, see Polyester Film Encapsulation, Library of Congress (1980). VincentDaniels, “The Elimination of Bleaching Agents from Paper,” Paper Conservator, Vol. 1 (1976): 9–11. GlenGray, in “Determination and Significance of Activation Energy in Permanence Tests,” has shown that diffusion rates influence the degradation of paper in accelerated aging. (Preservation of Paper and Textiles of Historic and Artistic Value, Advances in Chemistry Series No. 164, John C.Williams, ed. (1977): 286–313, p. 289.) For recent discussions of accelerated age testing, see E.E.Graminski, E.J.Parks, and E.E.Toth, “The Effects of Temperature and Moisture on the Accelerated Aging of Paper,” Restaurator, Vol. 2 (1978): 175–178; and W.K.Wilson and E.J.Parks, “An Analysis of the Aging of Paper. Possible Reactions and Their Effects on Measurable Properties,” Restaurator, Vol. 3 (1979): 38–62. In the latter, Wilson and Parks point out that while ideal accelerated aging conditions are as yet impossible to define, an accelerated aging test does provide information for ranking different samples with regard to specified criteria. Tinius Olsen Testing Machine Co., Instruction Booklet No. 64-10 for Folding Endurance Tester, p. 12–13, lists procedure according to ASTM Specification D-2176-63T. This differs from TAPPI T-511 su-59 in not requiring a fan to cool the oscillating head. Standard deviation for all data groups was calculated at the 90% confidence level with t values from W.L. Masterton and E.J. Slowinski, Mathematical Preparation for General Chemistry, W. B. Saunders Co., London (n.d.): p. 176, according to directions for calculation of population standard deviation, The HP-19C Printing and HP-29C Programmable Scientific Calculators Owner's Handbook and Programming Guide, Hewlett-Packard Co. (September 1977): p. 87–89, using the following formula, where “n” is the number of data points and “σx” is the sum of the values of the data points:
Instron Operating Instructions, Manuals No. 10-13-1M(D), 10-13-2 (TM-M), 10-2-2(C), 10-32-5(C). Munsell Book of Color. Glossy Finish Collection with Removable Samples. Munsell Color Co., (1966). Bausch & Lomb Instructions Book No. 332961-311ND. Beckman Instructions 81689-A. Ingold Electrode No. 18513, General Information Booklet.
This procedure is adapted from the description by Richard D.Smith in his article “A Comparison of Paper in Identical Copies of Books,” Restaurator, Supplement No. 2 (1972), p. 22–23. GlenGray, op. cit., states that when internal sizing agents have not completely polymerized in the manufacturing process, strength factors may increase during initial phases of the incubation cycle. Similar non-linear aging behavior has been reported for more complex adhesive-paper systems in which the components of the system experience marked alteration during the aging process, e.g., degradation, cross-linking. (N.S.Baer, N.Indictor, and W.H.Phalen, “An Evaluation of Adhesives for Use in Paper Conservation,” Journal of the Guild of Bookworkers, Vol. 10, No. 1 (1971): 17–35.) Tang and Jones, op. cit., p. 77–78. MargaretHey, “The Washing and Aqueous Deacidification of Paper,” The Paper Conservator, Vol. 4 (1979): 66–80. Tang and Jones, op. cit., p. 78. ACKNOWLEDGEMENTSTHIS RESEARCH WAS UNDERTAKEN in a course given at the Conservation Center of the Institute of Fine Arts, New York University. The authors thank the Co-Chairmen of the Conservation Center, Profs. N. S. Baer and L. J. Majewski, for their support, and also Mrs. Violet Bourgeois and Ms. Lee Stoby. Thanks are due also to the other members of the class for their valuable suggestions and criticism: Jeanne Brako, Deborah Trupin, Dianne O'Neal, and Ellen Pearlstein.
 Section Index Section Index |