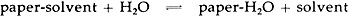THE RETENTION OF ORGANIC SOLVENTS IN PAPERJ. S. Arney, & L. B. Pollack
3 RESULTS AND DISCUSSIONSAMPLES OF WHATMAN'S #1 PAPER, a commercial rag paper, and a commercial newsprint were soaked for 15 minutes in open beakers containing organic solvents typically encountered in a conservation laboratory. After soaking, the paper samples were dried and analyzed for solvent content using the gas chromatographic procedure described in the Experimental portion of this report. The results shown in Table I indicate that solvent-paper complexes are formed as a result of solvent washing, as suggested by W�chter2 and as reported by others.3–11 Moreover, the complexes were found to be stable when exposed to anhydrous drying conditions, also in agreement with results already published. For example, ethanol was retained in Whatman's #1 even after 16 hours at 105�C in a ventilated oven. However, of more interest in conservation is the observation that none of the solvents were retained in any of the papers exposed overnight at 20�C to moisture-containing air, as shown in Table II. TABLE I SOLVENT RETENTION IN PAPERS WASHED 15 MINUTES IN SOLVENTS AND DRIED AT 0% RH TABLE II SOLVENT RETENTION IN PAPERS WASHED 15 MINUTES IN SOLVENTS AND DRIED AT 50% RH, 20�C The results reported in Table I show that stable inclusion complexes can easily be formed simply by washing papers in organic solvents. However, the term “stable” must be understood in the context of the experiment performed. Many researchers have used the term “stable” to refer to the resistance of solvent-cellulose complexes to decompose under the influence of heat. Wade and Creely,8 for example, reported that some of these complexes are stable at temperatures as high as 200�C. However, these complexes are not stable with regard to moisture. Several researchers have reported that bathing these complexes in water results in a complete displacement of the solvent.8 The results reported in Table II demonstrate that moisture in the air is also able to displace solvents trapped in solvent-washed papers. The interaction between paper and solvents can be understood as an equilibrium, as shown in the following scheme.
A particularly interesting case is thymol, often used as a fumigant. Samples of rag paper and newsprint were exposed to thymol fumes in a 40�C chamber for a week. Analysis revealed the sorption of significant amounts of thymol, as shown in Table III. However, after 24 hours at 20�C, 50% RH, no thymol could be detected. Thus, it would appear that thymol, like an organic solvent, can participate in the equilibrium shown in the above scheme. TABLE III THYMOL CONTENT OF RAG AND NEWSPRINT PAPERS AFTER 7 DAYS' EXPOSURE TO THYMOL VAPOR AT 40�C FOLLOWED BY EXPOSURE TO AIR AT 50% RH, 20�C |