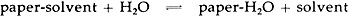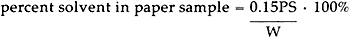THE RETENTION OF ORGANIC SOLVENTS IN PAPERJ. S. Arney, & L. B. Pollack
ABSTRACT—The retention of organic solvents in solvent-treated papers has been explored using gas chromatography. Results suggest that moisture in the atmosphere will displace retained solvents completely in a few hours under an ordinary environment. A related study of the sorption of thymol vapors by paper indicates that thymol also is not retained permanently in paper. 1 INTRODUCTIONTHERE HAVE BEEN REPORTS in the conservation literature that suggest the rate of aging of some papers may be accelerated by treatment with organic solvents. For example, Eirk1 reported that the degree of degradation of an old ledger paper after five days at 98–99�C (50% RH) was increased by bathing the paper in organic solvents. Moreover, W�chter,2 in a review of some early work by Staudinger,3,4 suggested that the treatment of a paper with an organic solvent might result in the formation of a permanent cellulose-solvent complex. According to W�chter, the formation of such a complex might increase the reactivity of the paper and accelerate its rate of aging. Whether, in fact, solvent treatment will accelerate paper aging has not been established, but the importance of solvents in the practice of conservation has prompted our investigation of some of the interactions between paper and solvents. The results of this investigation suggest that organic solvents are not permanently retained in cellulose under ordinary conditions and, therefore although there may be hazardous aspects to the use of solvents on paper, solvent retention alone cannot be regarded as potentially harmful. 2 SOLVENT-CELLULOSE COMPLEXESIT HAS LONG BEEN KNOWN that organic solvents, under some conditions, can form non-stoichiometric complexes with cellulose. Mease,5 in 1933, first noted the phenomenon as an anomalous weight gain in analytical filter paper washed with ethanol. This weight gain, as high as 2.8%, was shown to cause significant errors in gravimetric analytical procedures. Other researchers have since demonstrated that virtually any organic solvent, under appropriate conditions, can be made to form complexes with cellulose.6,7,8 Moreover, many of these complexes have been shown to be stable for long periods of time at elevated temperatures or under a high vacuum.6,7,8 The highest levels of solvent retention reported in the literature have been achieved by pre-swelling the cellulose in water, or some other swelling agent, followed by a liquid exchange with an organic solvent.9,10,11 Complexes formed in this way not only retain significant amounts of solvent (as high as 10–20%), but also retain much of the swollen fiber structure of the original water-wet cellulose.8 Moreover, these cellulose complexes have been shown to be of a higher chemical reactivity in heterogeneous reactions such as hydrolysis and acetylation.8 Thus, W�chter's concern that cellulose-solvent complexes might be formed when papers are brought in contact with solvents during conservation treatment appears to be justified. If such complexes are formed and do not decompose, then one would indeed expect papers treated with organic solvents to be more reactive and to age more quickly. 3 RESULTS AND DISCUSSIONSAMPLES OF WHATMAN'S #1 PAPER, a commercial rag paper, and a commercial newsprint were soaked for 15 minutes in open beakers containing organic solvents typically encountered in a conservation laboratory. After soaking, the paper samples were dried and analyzed for solvent content using the gas chromatographic procedure described in the Experimental portion of this report. The results shown in Table I indicate that solvent-paper complexes are formed as a result of solvent washing, as suggested by W�chter2 and as reported by others.3–11 Moreover, the complexes were found to be stable when exposed to anhydrous drying conditions, also in agreement with results already published. For example, ethanol was retained in Whatman's #1 even after 16 hours at 105�C in a ventilated oven. However, of more interest in conservation is the observation that none of the solvents were retained in any of the papers exposed overnight at 20�C to moisture-containing air, as shown in Table II. TABLE I SOLVENT RETENTION IN PAPERS WASHED 15 MINUTES IN SOLVENTS AND DRIED AT 0% RH TABLE II SOLVENT RETENTION IN PAPERS WASHED 15 MINUTES IN SOLVENTS AND DRIED AT 50% RH, 20�C The results reported in Table I show that stable inclusion complexes can easily be formed simply by washing papers in organic solvents. However, the term “stable” must be understood in the context of the experiment performed. Many researchers have used the term “stable” to refer to the resistance of solvent-cellulose complexes to decompose under the influence of heat. Wade and Creely,8 for example, reported that some of these complexes are stable at temperatures as high as 200�C. However, these complexes are not stable with regard to moisture. Several researchers have reported that bathing these complexes in water results in a complete displacement of the solvent.8 The results reported in Table II demonstrate that moisture in the air is also able to displace solvents trapped in solvent-washed papers. The interaction between paper and solvents can be understood as an equilibrium, as shown in the following scheme.
A particularly interesting case is thymol, often used as a fumigant. Samples of rag paper and newsprint were exposed to thymol fumes in a 40�C chamber for a week. Analysis revealed the sorption of significant amounts of thymol, as shown in Table III. However, after 24 hours at 20�C, 50% RH, no thymol could be detected. Thus, it would appear that thymol, like an organic solvent, can participate in the equilibrium shown in the above scheme. TABLE III THYMOL CONTENT OF RAG AND NEWSPRINT PAPERS AFTER 7 DAYS' EXPOSURE TO THYMOL VAPOR AT 40�C FOLLOWED BY EXPOSURE TO AIR AT 50% RH, 20�C 4 CONCLUSIONSIT WOULD BE HAZARDOUS to conclude from this research that solvents are entirely safe for use on paper. It is perhaps an overgeneralization even to conclude that no paper will ever retain a solvent permanently. However, the results of this investigation do indicate that most ordinary solvents will not be retained by the cellulose in paper under a normal museum environment. Thus, if solvent treatment accelerates paper aging in general (a phenomenon not yet established), then such an effect coult not be attributed to the formation of a permanent cellulose-solvent complex. Thymol is of particular interest in the context of retained organic matter. If thymol is not retained in papers, its fungicidal activity would be lost. Thus, a thymol treatment might inhibit the active growth of mold, but prolonged protection from mold growth would not be expected. The retention of thymol would not be desirable in any case, however, because thymol suffers oxidative decomposition to a brown, oily material in a relatively short time. This phenomenon, often observed to occur in trays of thymol used in treatment chambers, would be a problem only if significant levels of thymol were retained in papers for prolonged periods. Although it is possible that a long-term decomposition of thymol might occur within some papers, particularly those treated with thymol-alcohol solutions, the problem is not expected to be of significance in the usual vapor-treatment process, particularly if the treated paper is thoroughly vented in a moist (50% RH) atmosphere. 5 EXPERIMENTALMaterials: The rag and newsprint papers were commercial papers with cold extraction pH values of 6.7 and 5.0 respectively. The solvents were Fisher, Inc. reagent grade. Solvent Treatment: Oven-dried samples of paper (45 to 55 mg) were conditioned for 72 hours at 50% RH, 20�C, and placed in beakers containing 25 ml of solvent. After 15 minutes, the samples were removed from the beakers, blotted to remove excess solvent, and dried under the conditions described in Tables I and II.
Solvent Analysis: Although a gravimetric method of analysis is often used to determine the solvent content of cellulosic materials,3–11 such an analysis is applicable only under anhydrous conditions. At 50% RH, both solvent and moisture can contribute to the weight of a paper sample. Thus, we chose an analytical The paper sample to be analyzed was place in a glass tube fitted with a septum cap, and 150 μl of an extraction solvent, containing 1.00% by volume of an internal standard, was added. The tube was allowed to condition for 48 hours at room temperature to insure complete extraction of the solvent from the paper.6 Following this extraction period, a sample of the solution (about 100 μl) was withdrawn from the tube and injected onto a 15 foot Carbowax 20M column. Table IV shows details of the analysis for each solvent. TABLE IV ANALYTICAL SYSTEMS FOR SOLVENT DETERMINATION WITH A 15 FOOT CARBOWAX 20M GC COLUMN Peak heights for the solvent and the internal standard were measured from the GC trace, and the percent solvent content of the original paper sample was calculated as follows.
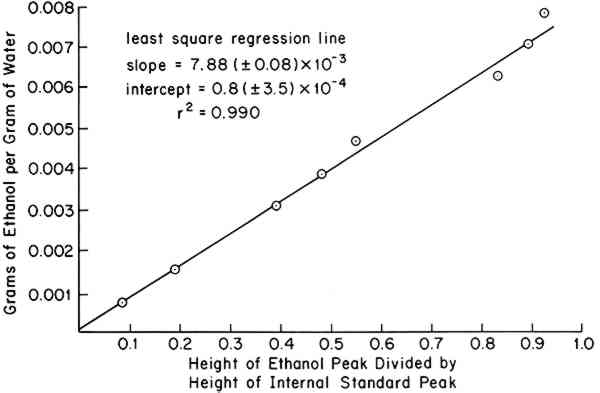
Sensitivity and Precision: Figure 1 shows a typical calibration curve obtained from 100 μl injections of standard solutions. The least-squares intercept was found to ACKNOWLEDGEMENTSTHIS RESEARCH was supported by a grant from the Andrew W. Mellon Foundation. REFERENCESK. G.Eirk, Bull. Amer. Group-IIC, 12(2), 82 (1972). O.W�chter, Mitteilungen, 4(4), 189 (1974). H.Staudinger, Naturwissenschaften, 42, 228 (1955). H.Staudinger, Zeitschrift f�r Angewandte Chem., 64, 149 (1952). R. T.Mease, Ind. and Eng. Chem., 5, 317 (1933). J.Wiertelak and I.Garbaczowna, Ind. and Eng. Chem., 7, 110 (1935). H.Staudinger, Makromol. Chem., 10, 254 (1953). R. H.Wade and J. J.Creely, Text. Res. J., 44, 941 (1974). M. V.Merchant, Tappi, 40(9), 771 (1957). G. A.Richter, L. E.Herdle and W. E.Wahtera, Ind. and Eng. Chem., 49, 907 (1957). J. K.Russell, O.Maass and W. B.Campbell, Can. J. Res., 15, Sec. B, 13 (1936).
 Section Index Section Index |