IDENTIFICATION OF DYES ON OLD TEXTILESHelmut Schweppe
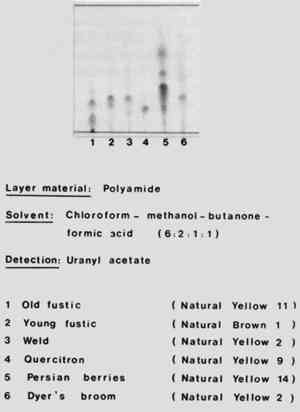
10 THIN-LAYER CHROMATOGRAPHYTHIN-LAYER CHROMATOGRAPHY can be carried out with minute samples, needs little apparatus, and is quick. If a natural dye contains several red-colored substances, thin-layer chromatography should be the method of choice. This is particularly true of identifying hydroxyflavone and hydroxyanthraquinone dyes. My experience has been that for the separation of natural dyes the best material for the stationary phase is polyamide powder. Figure 5 shows the chromatograms of various yellow natural dyes of the hydroxyflavone class. Polyamide was used for the stationary phase, and the mobile phase consisted of a mixture of chloroform, methanol, butanone, and formic acid in the proportions by volume of 6:2:1:1. To show up the spots the chromatogram was sprayed with a solution of uranyl acetate. The chromatograms shown include those of old fustic, young fustic, weld, quercitron, Persian berries, and dyers' broom. the two natural dyes weld (no. 3) and dyers' broom (no. 6) contain luteolin as the principal coloring material, and are included in the Color Index as Natural Yellow 2.
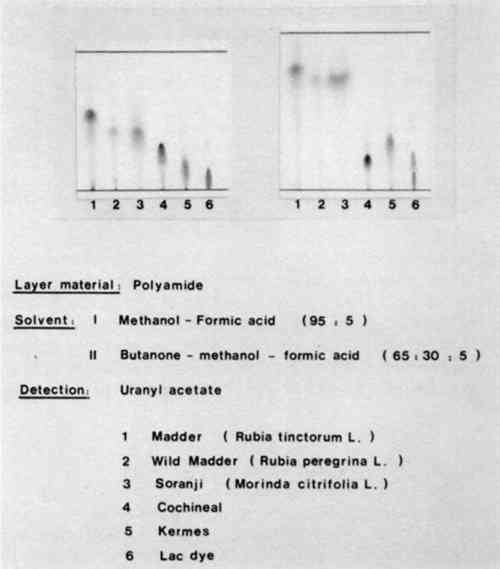
Figure 6 shows the chromatogram of natural red dyes containing hydroxyanthraquinones. The stationary phase was polyamide but two different mobile phases were used. The chromatograms on the left were developed with a mixture of methanol and formic acid in the ratio 95:5, while those on the right were developed with a mixture of butanone, methanol, and formic acid in the proportions 65:30:5. Both sets of chromatograms include madder, wild madder, soranji (a type of Indian madder), cochineal, kermes, and lac dye. You can see that the first mobile phase madder and wild madder, and cochineal and lac dye can be differentiated.
An example of the use of this method was the investigation of a piece of satin made in Italy about 1500 A.D. By thin-layer chromatography on polyamide it could be shown that both carminic and kermesic acids were present, indicating, that the dye used was Polish kermes, since this is the only dye containing both these acids. Another example was the study of a piece of an old Ushak carpet said to have been made about 1600. The various dyes were identified by means of their colored lakes. The red was madder, the brownish-yellow weld, and the green was also shown to be weld after the material had been extracted with dimethylformamide. The results obtained by forming the colored lakes were confirmed by thin-layer chromatography. Chromatograms of the dyes from the red, yellow, and green knots showed clearly that the chromatogram of the red is identical with that of madder and that the yellow and the yellow component of the green agree with the chromatogram of weld. A further case was furnished by the examination of a piece of a 19th century Kazak carpet with two shades of red, which we will call red 1 and red 2. Red 1 was identified as a madder dye, while red 2 was an acid red azo dye. Boiling with dilute ammonia had almost no effect on red 1, only traces of red going into solution. Red 2 however could be almost quantitatively removed from the fibre with ammonia. Red 1 could be identified as madder by means of comparison of the This last example in particular demonstrates that very useful results can be obtained without employing very elaborate equipment or methods. |