IDENTIFICATION OF DYES ON OLD TEXTILESHelmut Schweppe
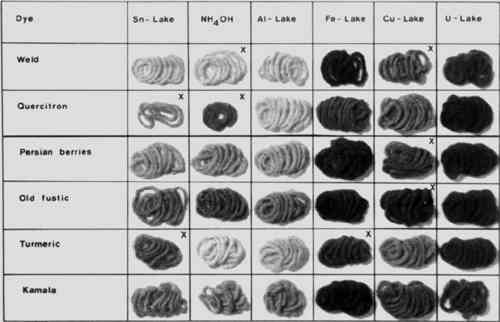
ABSTRACT—A method for the differentiation of natural from synthetic dyes on textiles is discussed, and a simple method for the identification of natural dyes on old textiles is established. Identification is accomplished by the preparation of lakes of the dyes and their color comparison with lakes of known dyes. Thin-layer chromatography for the identification of certain red dyes and yellow dyes is also discussed, and several examples of identification of unknown dyes are described. 1 INTRODUCTIONONLY A FEW EXPERTS are capable of identifying dyes on old textiles, because most of the methods currently used require expensive apparatus, are time-consuming, and can be evaluated only by people with much experience, or because the necessary collection of authentic samples is not available. I wish to introduce here a method of analysis that is simple, quick, and cheap and is suitable for identifying the most important natural dyes on textiles. However, even this method cannot be carried out without making color comparisons, and I have therefore included a list of suppliers of natural dyes and books on dyeing with natural dyes (Appendices 1 and 2). In contrast to methods previously known, I identify the natural dyes on the fibres, without taking them up into solution. Many of the natural dyes are mordant dyes, present on the fibre as insoluble lakes, for example aluminum lakes. In such cases one can form different lakes by boiling with aqueous solutions of tin, aluminum, iron, copper, or uranium salts, and then one obtains a series of lakes of different shades. By making comparisons with known dyeings it is very often possible to make an unambiguous identification of particular natural mordant dyes. This method of analysis is really based on the method of dyeing whereby the color is first developed on the fibre. This method of dyeing with mordant dyes is used when a metal salt is not suitable for direct mordanting of the fibre. For instance, one can dye wool mordanted with alum and then treat it with an aqueous solution of, say, copper sulfate, forming the copper lake on the fibre. I shall give a short sketch of the method of analysis and then enlarge on this with the aid of specific examples. However, I should first like to mention some preliminary tests, which in some cases can give important indications. 2 PRELIMINARY EXAMINATIONA SMALL SAMPLE of the colored fabric is first boiled in a 1% ammonia solution, in order to remove soil and finishes. Most natural dyes do not run when subjected to this treatment, since they are usually mordant dyes, that is to say they are present in the fabric as insoluble lakes. Indigo is also fast to dilute ammonia. Most of the earlier synthetic dyes made prior to the end of the 19th century run considerably. The same observation is made by textile restorers when they wash carpets with anionic surfactants in ammoniacal solution: if they see that no dye runs they draw conclusions about the presence of natural or synthetic dyes. Prior to testing the fabric sample is washed with water and then with methanol, pressed between filter papers and set aside to dry. A small piece of the cleaned dyeing is boiled successively with water, ethanol, Most of the natural dyes are extracted only slightly or not at all. There are a few exceptions however, one of which is provided by safflower, whose most important color constituent is carthamic acid. The red carthamic acid loses its color irreversibly when boiled with ammonia, while the safflower yellow that accompanies it goes into solution without change in color. Indigo extract is also extracted to a considerable extent by boiling ammonia. This dye, which has been made by treating indigo with sulfuric acid since 1740, consists principally of indigo-disulfonic acid. When a synthetic dye is believed to be present because the color is extracted by water and ammonia, or ethanol and glacial acetic acid, simple dyeing tests can be used to indicate whether it is an acid, basic or direct dye. The most strongly colored extract is evaporated to dryness and the residue is taken up in a little water. The solution is divided in two portions; one is acidified with acetic acid, and to part of the other (5 ml) a 5% sodium sulfate solution (1 ml) is added. The solutions are tested on wool and tannin-mordanted cotton.2 if the wool is stained more strongly by the acid solution than the cotton, an acid dye is present. If the cotton is dyed more strongly, a basic dye is present. A direct dye can be recognized by the fact that it easily goes onto unmordanted cotton from the neutral solution containing sodium sulfate. Two simple tests can be used to confirm the presence of synthetic dyes. If the ammonia extract is strongly colored and the color disappears when the extract is shaken with a little zinc powder at room temperature, the dye is an azo dye. If a few drops of concentrated sulfuric acid are poured over a small portion of a dyed fabric and observed for a few minutes, the acid may develop an intense color (red-violet, blue, or green); and if it does so, this is a certain indication of synthetic dye. 3 COLOR REACTIONS FOR THE IDENTIFICATION OF NATURAL MORDANT DYESTHE PRINCIPLE of the reactions described here is that natural mordant dyes form lakes of various colors with tin, aluminum, iron, copper, and uranium. In addition, treatment of the tin lakes with 20% ammonia solution sometimes results in very striking color changes that can lead to the identification of certain dyes, such as orchil or sandalwood. The complete set of six colors is usually unique and provides a rapid and simple means for identifying many natural mordant dyes, provided of course that comparison sets obtained from authentic samples are available. It is usual to form the tin lake first, since treatment with a strong solution of stannous chloride destroys the color of most acid and direct azo dyes and of acid metal-complex dyes, thus providing an additional indication that a synthetic dye is present. The stannous chloride reagent is prepared by dissolving stannous chloride dihydrate (1 g) in concentrated hydrochloric acid (1 ml) and diluting with water (4 ml). The procedure is to take a small portion of the fabric that has been cleaned with dilute ammonia solution, boil it in the stannous chloride reagent, allow it to stand in the reagent for 10 minutes, wash it thoroughly with water (until the washings are neutral) and divide it into halves. Drying the treated material for this and the The other lakes are formed similarly by treating four separated portions of the clean sample with 2% aqueous solutions of alum, ferrous sulfate, copper sulfate, and uranyl acetate. As in the formation of the tin lake, the solutions are boiled and then allowed to stand for 10 minutes before the material is washed with water. The following figures illustrate the various lakes formed by natural dyes of more than local importance that are, or were, used for dyeing textiles. The dyes are grouped by color, since this is the most obvious classification for the analyst. 4 YELLOWFIGURE 1 shows the lakes formed by the yellow natural dyes that are most frequently encountered: weld extract, quercitron, Persian berries, old fustic, turmeric, and kalama. Shown are the colors of the aluminum, iron, copper, and uranium lakes of the dyes just mentioned, and also the colors of the tin lakes both before and after treatment with ammonia. In practice the dyes are usually in the form of the aluminum lake in the fabric, so that boiling with alum solution seldom changes the yellow shade, except perhaps to brighten it through soil removal. The lakes whose colors differ distinctly from the others are marked with a cross.
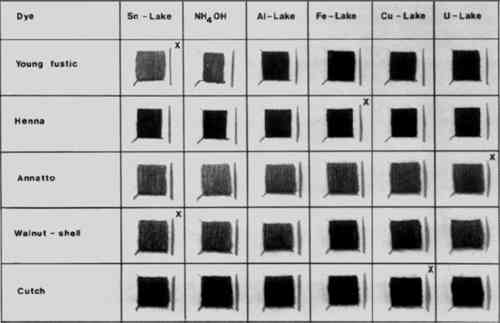
Looking at Figure 1 one can see that only the tin lakes of quercitron and turmeric are orange. When the tin lake of turmeric is treated with ammonia it turns brown at first and then becomes yellow when washed with water. That of quercitron turns deep orange when heated with ammonia. These observations are sufficient to distinguish the two dyes from the other four. Old fustic and kamala both have orange-yellow tin lakes, but can be distinguished by the behavior of these lakes when treated with ammonia and also by the colors of their iron and copper lakes. Weld extract and Persian berries both have yellow tin lakes, but can be distinguished particularly by the behavior of these lakes when treated with ammonia and by the different colors of their copper lakes. 5 ORANGE AND BROWNFIGURE 2 shows the lakes of the commonest orange/brown natural dyes. These include young fustic (the most commonly-encountered orange natural dye), henna, annatto, walnut-shell, and cutch. Here too you can see that the colors of the individual lakes can be very different. Henna dyeings can be distinguished by the color of the iron lake, and annatto dyeings by the color of the uranium lake.
An important point to note about brown fabrics is that they are sometimes made from brown wool, and scarcely change color when treated with the various metal salts. 6 RED
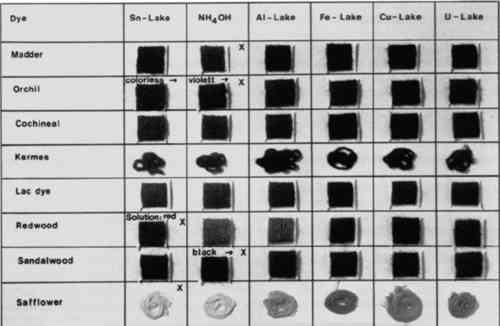
FIGURE 3 shows the lakes of the most important red natural dyes: madder (the most commonly-encountered natural red dye), orchil, cochineal, kermes, lac dye, redwood, sandalwood, and safflower. Madder can be recognized by its orange tin lake. Orchil can be identified with certainty by its failure to form colored lakes with tin and the intense violet formed on subsequent treatment with ammonia. Redwood
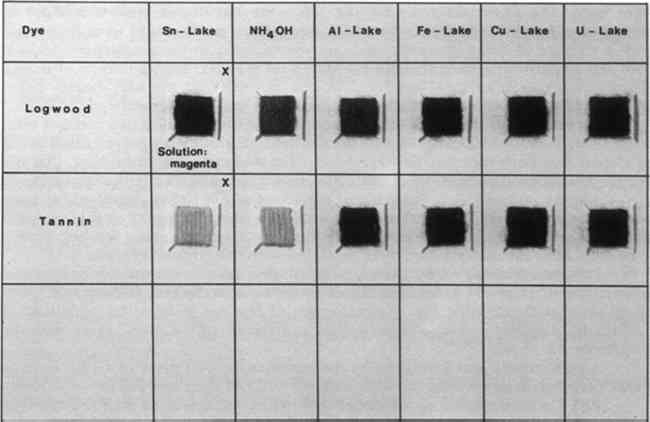
7 BLACKTHE COLORS OF THE LAKES formed by the two natural black dyes logwood and tannin are shown in Figure 4. The two types of black can be distinguished unambiguously by treatment with stannous chloride. Logwood blacks change to violet, and the solution is colored an intense magenta. Tannin blacks lose their color when boiled with stannous chloride; the color is not restored on treatment with ammonia. They also lose their color when treated with 10% sulfuric acid.
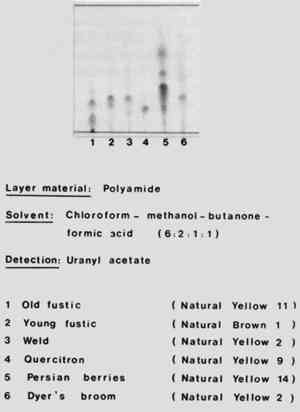
8 MIXTURES OF NATURAL DYES AND INDIGOIF THE FABRIC has a green or violet shade obtained by the use of natural dyes,and in the preliminary test a blue color is extracted by boiling glacial acetic acid, one should test for indigo by the method described by Hofenk-De Graaff (“A Simple Method for the Identification of Indigo,” Studies in Conservation, v. 19, [1974]: 54–5). If the result of the test is positive the indigo should be removed from the fibre by repeated boiling with dimethylformamide until the solvent remains colorless. Once the indigo has been removed the yellow component (in green dyeings) or the red component (in violet dyings) is left behind and can be identified by forming the various colored lakes as described above. An interesting example of the method just described was the examination of a fragment of Coptic fabric dating from the 6th centruy that had been dyed with indigo and madder, a mixture that has been called Egyptian purple. The color of the original sample was dark violet. Treatment with ammonia did not change the color but extraction with dimethylformamide took out the indigo, leaving the red on the fibre. The colored lakes formed by the red dye matched those of the red obtained from wild madder (Rubia peregrina). It was confirmed by thin-layer chromatography that ordinary madder from Rubia tinctorum was not present, since only purpurin (1, 2, 4-trihydroxy-anthraquinone) was found: alizarin (1, 2-dihydroxyanthraquinone) was absent. 9 RAPID TEST FOR DISTINGUISHING AMONG MADDER, COCHINEAL, AND KERMESONE TAKES A SMALL PORTION of the red dyeing, adds a few drops of concentrated sulfuric acid, and waits for a few minutes until the dye has colored the acid. Madder gives a dull red solution that fluoresces orange in ultraviolet light; cochineal (and lac dye) gives a magenta solution; kermes gives a dull red-violet solution. If a few milligrams of boric acid are then added to the sulfuric acid, color changes may be observed. If cochineal (or lac dye) is present the color changes to blue; if kermes is present the color changes to brownish-violet; if madder is present there is no change in color. If the solution is diluted with about ten times its volume of water and shaken with a The ether and pentanol extracts can be washed throughly with water to remove acid and used for identification of the dyes by means of thin-layer chromatography. The latter is the only simple way by which one can distinguish between cochineal and lac dye. 10 THIN-LAYER CHROMATOGRAPHYTHIN-LAYER CHROMATOGRAPHY can be carried out with minute samples, needs little apparatus, and is quick. If a natural dye contains several red-colored substances, thin-layer chromatography should be the method of choice. This is particularly true of identifying hydroxyflavone and hydroxyanthraquinone dyes. My experience has been that for the separation of natural dyes the best material for the stationary phase is polyamide powder. Figure 5 shows the chromatograms of various yellow natural dyes of the hydroxyflavone class. Polyamide was used for the stationary phase, and the mobile phase consisted of a mixture of chloroform, methanol, butanone, and formic acid in the proportions by volume of 6:2:1:1. To show up the spots the chromatogram was sprayed with a solution of uranyl acetate. The chromatograms shown include those of old fustic, young fustic, weld, quercitron, Persian berries, and dyers' broom. the two natural dyes weld (no. 3) and dyers' broom (no. 6) contain luteolin as the principal coloring material, and are included in the Color Index as Natural Yellow 2.
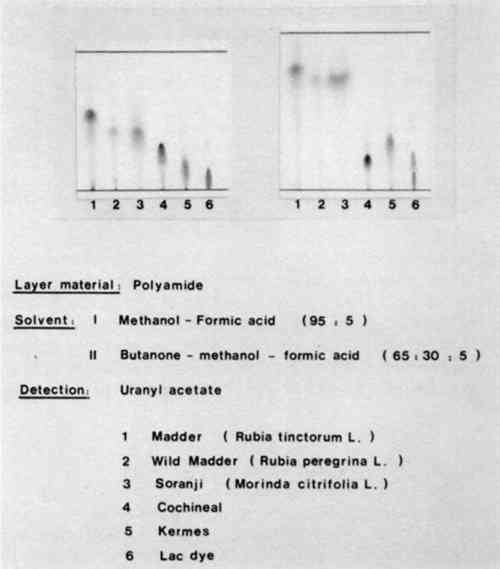
Figure 6 shows the chromatogram of natural red dyes containing hydroxyanthraquinones. The stationary phase was polyamide but two different mobile phases were used. The chromatograms on the left were developed with a mixture of methanol and formic acid in the ratio 95:5, while those on the right were developed with a mixture of butanone, methanol, and formic acid in the proportions 65:30:5. Both sets of chromatograms include madder, wild madder, soranji (a type of Indian madder), cochineal, kermes, and lac dye. You can see that the first mobile phase madder and wild madder, and cochineal and lac dye can be differentiated.
An example of the use of this method was the investigation of a piece of satin made in Italy about 1500 A.D. By thin-layer chromatography on polyamide it could be shown that both carminic and kermesic acids were present, indicating, that the dye used was Polish kermes, since this is the only dye containing both these acids. Another example was the study of a piece of an old Ushak carpet said to have been made about 1600. The various dyes were identified by means of their colored lakes. The red was madder, the brownish-yellow weld, and the green was also shown to be weld after the material had been extracted with dimethylformamide. The results obtained by forming the colored lakes were confirmed by thin-layer chromatography. Chromatograms of the dyes from the red, yellow, and green knots showed clearly that the chromatogram of the red is identical with that of madder and that the yellow and the yellow component of the green agree with the chromatogram of weld. A further case was furnished by the examination of a piece of a 19th century Kazak carpet with two shades of red, which we will call red 1 and red 2. Red 1 was identified as a madder dye, while red 2 was an acid red azo dye. Boiling with dilute ammonia had almost no effect on red 1, only traces of red going into solution. Red 2 however could be almost quantitatively removed from the fibre with ammonia. Red 1 could be identified as madder by means of comparison of the This last example in particular demonstrates that very useful results can be obtained without employing very elaborate equipment or methods. APPENDIX1 APPENDIX 1: SUPPLIERS OF NATURAL DYES/DRUGSPaul M�ggenburg, Drogen and Vegetabilien, Einfuhr- und Ausfuhrhandel, Wandalenweg 24, 2 Hamburg 1, West Germany Petereit & Co., Medizinaldrogen, Import-Export, Groβhandlung Flamweg 132/134, Postfach 852, 22 Elmshorn bei Hamburg, West Germany Hellmuth Carroux, Import-Export, Drogen-Talkum-Schwefel-Gummiharze-Schellack-Kernmehle, Neuer Wall 37, 2 Hamburg 36, West Germany C. E. Roeper, Fach-Importeur, Drogen-Harze-Quellstoffe, Klosterallee 74, 2 Hamburg 13, West Germany Etablissements A. Longeval S.A., Herboristerie en gros-Drogues-Produits chimiques-Essences-Gommes, Grand'Rue, 48, B-7870 Deux-Acren, Belgium Compagnie Fran�aise des Extraits Maison Westphalen, B.P. 1375, 20, Rue de Pressence, 76 Le Havre, France 2 APPENDIX 2: BOOKS ON COLORING WITH NATURAL DYESNatural Dyes and Home Dyeing (formerly titled: Natural Dyes in the United States). A practical guide with over 150 recipes. By Rita J. Adrosko. Dover Publications, Inc., 180 Varick Street, New York, N.Y. 10014, (1971). Natural Dyes. By Sallie Pease Kierstead. Boston Branden Press Publishers (1972). Dyes from Plants. By Seonaid M. Robertson. Van Nostrand Reinhold Company, New York-Cincinnati-Toronto-London-Melbourne (1973). Wir f�rben mit Pflanzen. Tagebuch eines F�rbelehrganges mit Beitr�gen von Irmgard Becker-Kutscher. Von Kurt Hentschel. WEBE MIT-Verlag, D-7065 Winterbach-Manolzweiler, West Germany (1977). F�rbbuch. Grundlagen der Pflanzenf�rberei auf Wolle. Von Emil Spr�nger. Eugen Rentsch Verlag, Erlenbach-Z�rich, Switzerland (1969).
Farben aus der Natur. Eine Sammlung alter und neuer Farbrezepte f�r das F�rben auf Wolle, Seide, Baumwolle und Leinen. Von Gretel Fieler. Verlag M. & H. Schaper, 3 Hannover, West Germany (1978). NOTES1. The 20% ammonia solution can be replaced by 25% ammonia solution (d = 0.91), which is the solution usually supplied commercially. 2. Dissolve tannic acid (0.6 g) in water (100 ml), heat the solution to 60–70�C, and immerse cotton yarn (10 g) in the hot solution. Set aside the whole for 2 hours until it is cool, then remove the cotton, squeeze it free of liquid, immerse it in a cold solution of tarter emetic (prepared by dissolving 0.3 g antimony potassium tartrate in 100 ml water) for 20–30 minutes, and finally rinse it thoroughly with water. After the yarn has dried in air small portions of it can be used for dying tests.
 Section Index Section Index |