THE RECOVERY OF COLOR IN SCORCHED OIL PAINT FILMSChristopher Tahk
ABSTRACT—The extent of darkening of various oil paint films on exposure to heat and the return of color attained by bleaching them with light have been investigated by reflectance spectrophotometry. Both long wavelength ultraviolet and visible radiation have been found to effect the recovery of paint color to an appearance which is often close to the original. In the cases studied, the degree of heat-induced darkening and subsequent color recovery during bleaching were different for each paint film, but most were substantially brightened by the action of the light. Various aspects of this potential tool for the conservation of fire-damaged oil paintings are discussed. CONSERVATION LITERATURE contains few accounts of the treatment of fire-damaged oil paintings. Articles1–4 have focused on methods of surface cleaning and consolidation with emphasis being placed on procedures for flattening blisters caused by the heat. Although the discoloration of paint by the heat of fire was occasionally noted, techniques for reversing this damage were not explored. This paper describes the results of an investigation into a method for recovering the original color of scorched oil paint films. Paint film scorching can cause different and complex physical and chemical changes in the medium, the pigment, or both. No attempt will be made here to identify in detail the specific reactions which may take place. At the outset of this study, however, it was anticipated that since many paints contain pigments which are more thermally stable than is the surrounding organic oil medium, the discoloration of the latter might often be entirely responsible for the observed discoloration of the scorched paint. The source of the discoloration of the medium would be light absorbing molecular groups (chromophores). Chromophores are often sensitive structures which can be selectively destroyed in the presence of more inert molecular groups by appropriate physical or chemical agents, these acting as bleaches. Assuming that this is true, the recovery of the original color of a scorched paint is then simply a matter of selecting a proper bleach and bleaching procedure which will eliminate chromophores in the medium and at the same time cause minimal damage to other paint components. Preliminary experiments suggested that light would be the simplest and most efficacious bleaching agent for a heat-discolored oil paint medium. This is not a new idea. Laurie5 cites a letter by Rubens advising that if an oil painting has been darkened by long storage out of the light its colors can be freshened by putting it “often in the sun.” Phillips6, 7 has had success using visible light to bleach newly uncovered wall paints darkened by long coverage under overlying paint layers. The lightfastness of most common oil pigments8 compared to the light sensitivity of many organic chromophores also encouraged the present study of this approach to the treatment of heat darkened paint. To obtain more information on the nature of the bleaching process, the following were investigated:
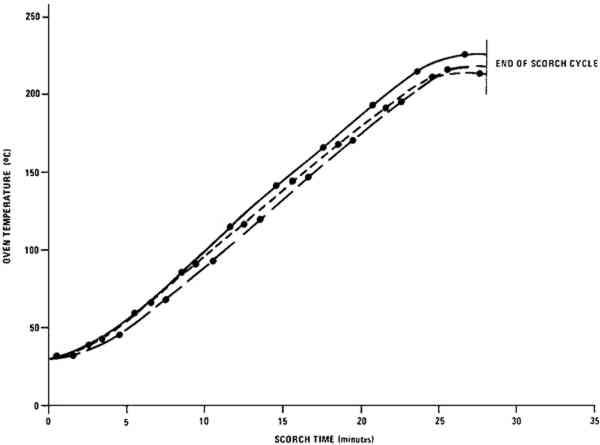
1 EXPERIMENTALPAINT SAMPLES were prepared from the commercial artists' oil tube colors listed in the Appendix.9 To obtain paint consistency suitable for layer preparation, the white samples were diluted with 15% of their weight by a 1:1 (v:v) mixture of linseed oil:turpentine.10 Each of the colored paints was thoroughly blended with 115% of its weight of the zinc white paint containing 35% of its weight of the 1:1 linseed oil:turpentine mixture. The paints were spread over one surface of 2 � 2 in., 0.5 mm thick aluminum panels using a 127 μm thick Mylar mask with a microscope slide as a drawbar to produce paint layers of an even thickness about equal to that of the mask. Prior to use, the coated squares were allowed to dry for three months under normal room lighting (indirect fluorescent lamp illumination for about twelve hours per day) while shielded from dust. Scorching of the paint samples was carried out in a laboratory oven11 equipped with an auxiliary heater and an internal fan to keep temperatures approximately equal throughout the oven chamber. Iron-Constantan thermocouples were used in conjunction with a strip-chart recorder to monitor temperatures at three different oven locations. The range of scorching conditions that caused color changes in the paint film but which did not carbonize the paint was determined empirically. One of
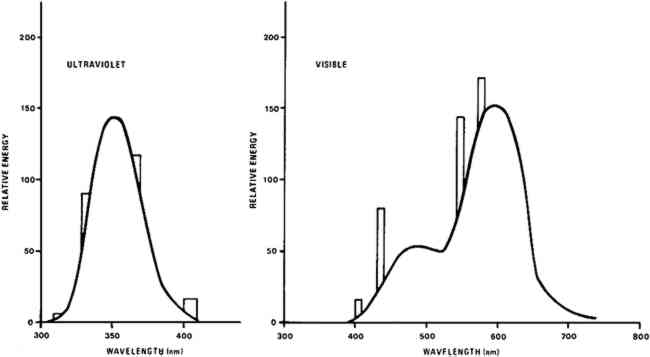
Light bleaching was effected under either a bank of General Electric (G.E.) 100 watt F84PG17-CW Power Groove Cool White fluorescent lamps or under a bank of G.E. 40 watt F40BLB “black light” lamps emitting in the near ultraviolet. The fluorescent light bank incorporated a one-eighth inch thick sheet of Rohm and Haas UF-3 Plexiglas which removed virtually all ultraviolet radiation emitted by the lamps. The typical spectral energy distribution of each lamp type, that of the cool white lamp corrected for the UF-3 filtration, is given in Figure 2.12 The measured intensity of the light from the visible light bank incident upon the paint samples was approximately 40,000 lux,13 about four hundred times greater than that on paintings on exhibit in many museums. No direct intensity measurements were made on the radiation produced by the long wavelength ultraviolet light bank. But, as these BLB fluorescent tubes have the same efficiency as an ordinary visible light producing fluorescent tube, about 22%,14 the intensity of the total radiant energy incident upon paint samples under the ultraviolet lamp bank is no more than 40% of that on samples under the bank emitting in the visible.
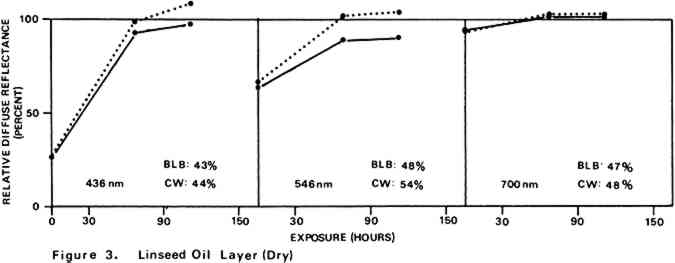
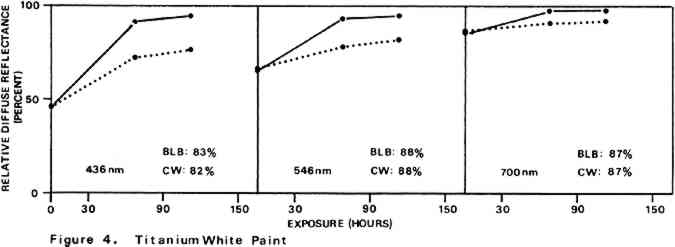
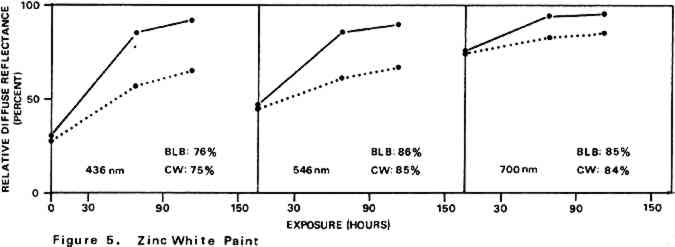
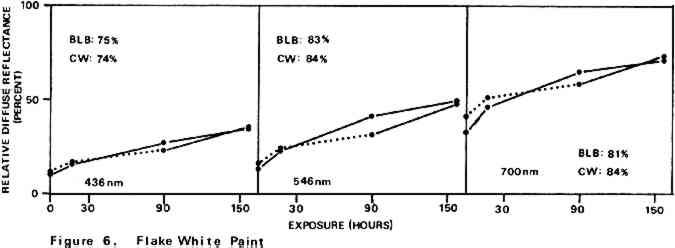
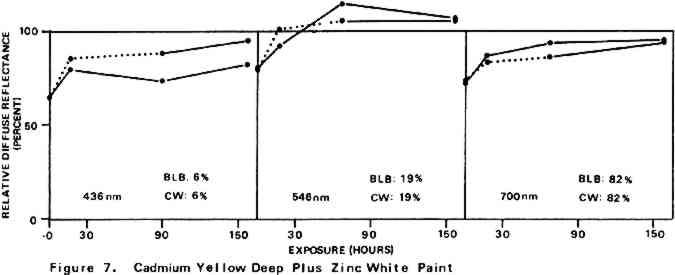
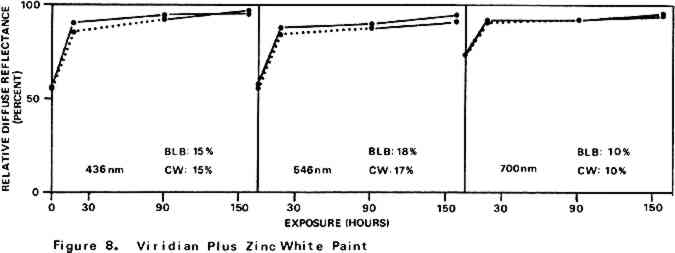
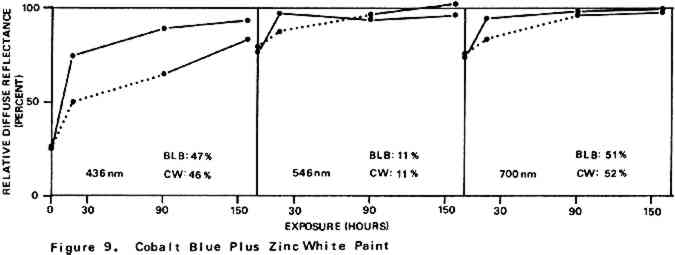
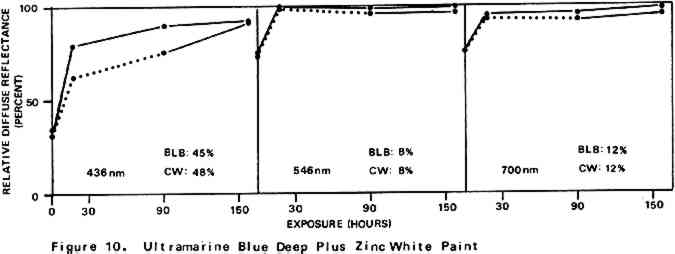
Environmental variables were not controlled during the experiments. Approximate ambient conditions, however, included temperatures of 25–30�C. and a relative humidity range of 55–75%. During bleaching the paint samples became only slightly warm to the touch. The paint sample layers were characterized by their diffuse reflectance spectra15 in the visible (375–750 nm) range as recorded with a Bausch and Lomb Spectronic 600 Spectrophotometer with an integrating sphere reflectance head. Measurements were made with instrument slit widths set to pass a 5 nm wide spectral band. Barium sulfate was employed as the reflectance standard. Use of a specially designed sample holder insured that each reflectance measurement on a given sample was always taken on the same part of the sample surface. The full reflectance spectra are not given here, but rather only the percent diffuse reflectance values at each of the three wavelengths of the three primaries of the 1931 CIE R,G,B color system: R (red), 700 nm; G (green), 546 nm; B (blue), 436 nm.16 Reflectance values at these three wavelengths serve as a gauge of the color of the paint. Only the three initial reflectance figures for each unscorched three-month-old paint layer are given as such: all others are presented as percentages of the initial reflectance value obtained on a given sample at a given wavelength, i.e., as relative diffuse reflectances. Recovery of original color through bleaching of a paint sample would result in all three relative diffuse reflectance values returning to 100%. 2 DISCUSSION AND RESULTS2.1 Scorching Characteristics of the Paint SamplesTHE EXTENT to which a paint sample darkened in a scorch cycle, as measured in terms of relative diffuse reflectance at each wavelength, is given by the first point in each of the reflectance-light exposure plots (Figures 3–10). Considerable differences in degree of color change which occurred in the paints are apparent. The variation in extent of darkening which can take place is perhaps most strikingly illustrated by the three whites. Titanium white is appreciably darkened, zinc white yet more so, and flake white is the most discolored, its 435 nm and 546 nm reflectance values being reduced to less than 20% of their original magnitude.
For almost all of the paint samples the relative decrease in reflectance observed in the scorch cycle at the three selected wavelengths is generally consistent with yellowing of the medium as the principal source of total color change. Such yellowing, most apparent in the case of the pure linseed oil film (Figure 3), results in greatest reflectance loss at the blue end of the spectrum and least at the red (i.e., relative reflectance decreases are greatest at 435 nm and least at 700 nm). The fact that different paints discolor to different extents under identical scorch conditions does not prove the pigments play a significant role in producing discoloration. For example, the yellow paint cadmium yellow deep will not suffer much color change if its medium becomes yellow whereas blue paints such as ultramarine blue will be darkened owing to absorption by the yellow medium of the blue light responsible for the paint's color. Too, the relative transparency and oil-to-pigment ratio of a paint will determine the length of the light path through its medium and, therefore, the extent to which a given degree of yellowing will alter original paint color. However, the blistering of flake white which took place only during the scorching of this paint strongly implies possible pigment involvement in the process. The blisters could have been caused by bubbles produced by the reaction of organic acids, created in the medium by heat, with the lead pigment to produce carbon dioxide gas and water 2.2 Light Bleaching of the Scorched Paint2.2.1 A. Summary of experimental results.The reflectance-light exposure plots for the paint samples show that all are significantly bleached during the exposure period by radiation from either the visible or the ultraviolet light banks, some color recoveries being particularly striking. Note that for visible light bleaching a twenty-five hour exposure equals approximately one million lux hours. A painting will receive a million lux hour exposure when on exhibit in a properly illuminated museum for no more than a few years. If, as is likely the case, the extent of bleaching is determined by total light exposure and not its intensity, then keeping a heat-darkened painting on exhibit rather than in storage out of the light is one way of noticeably improving its colors. The reflectance-light exposure plots for most paints show that the visible light bank with its UF-3 Plexiglas filter gives off negligible ultraviolet radiation and is more powerful in its bleaching action than is the ultraviolet bank. This comparison does not take into account the fact that radiation from the latter is about only 40% as intense on an energy basis. If intensity of radiation alone, not wavelength, determines bleaching rate, a scorched paint film should bleach about 40% as rapidly under the ultraviolet bank as it would under the visible. For a few paints (cobalt blue plus zinc white, ultramarine blue plus zinc white, and zinc white alone), even on an equal energy intensity basis, visible light appears to be the superior bleach, at least over the bleaching periods within which most of the scorched paints underwent the greatest extent of color recovery (see Figures 3–10). For other paints, visible and ultraviolet radiations at equal energy intensities are either comparable in effecting color recovery or the ultraviolet radiation appears superior. Whether this relative effectiveness of the two light sources holds up over longer exposure times has not been established, although preliminary, qualitative experiments carried out by the author suggest that it does. A reason for preferring visible to ultraviolet light bleaching in all cases is considered later in this section in a discussion of bleaching chemistry. Figures 3–10 also show that, with the exception of flake white paint, the rate of color recovery decreases with exposure time. In separate experiments involving longer light exposure periods, however, flake white paint has also been found to exhibit this bleaching behavior. While the data are insufficient to permit a specific relationship to be established between total exposure and degree of color recovery, it is apparent that perfect color recovery, if attainable at all in most appreciably scorched paints, would require extremely long bleach times. Certainly, in any practical application of this method in conservation, a compromise must be struck between the extent of color recovery realized and the length of light exposure time necessary to obtain it. Most of the exposure-reflectance charts also show that bleachings tend to give recovery of a color close to the original rather than producing another distinctly different in appearance. There are a few exceptions: in these, the relative reflectance values at each of the three wavelengths measured do not always return to the 100% relative reflectance value as exposure times increase. In the instance of cadmium yellow deep, the high 546 nm values are not easily explained except by assuming that some slight change in pigment color has occurred. This is in accord with Levinson's comment17 that cadmium yellows “have limited reliability if subjected to too much A study of the possible enhancement of light bleaching by exposing darkened samples first to one light bank and then to the other was unrewarding. Scorched paint samples which had received a ninety-one hour exposure under the ultraviolet lamp bank were exposed for an additional sixty-seven hours under the visible bank (see Figures 6–10). The final visible light exposure somewhat increased the extent of 2.2.2 B. Chemical aspects of scorched paint bleaching.Even though the specific bleaching reactions are unknown, the initial steps are certainly photochemical. Electronic excitation of a chromophore in the discolored medium by the absorption of a photon can result in chromophore destruction in one or more ways. Experiments in this laboratory have shown that bleaching by ultraviolet radiation of heat darkened films of all three white paints occurs less than half as rapidly in a helium atmosphere as it does in air. This indicates that the process is in part a photooxidation. Other experiments have established that, as expected, the blue end of the visible spectrum is responsible for most of the bleaching power of the visible light bank, wavelengths as long as 550 nm apparently contributing to chromophore destruction. The low energies of photons of visible light generally makes them less capable of inducing photochemical processes than are photons of the ultraviolet. Nonetheless, visible light photons have sufficient energy to convert, via the agency of a sensitizer, molecular oxygen in the paint film from the triplet to singlet electronic state. Singlet oxygen is becoming recognized as the causative agent in many photooxidations of organic materials.18 At least in bleachings effected with ultraviolet radiation, in one bleach reaction the pigment could play a role. In the presence of ultraviolet light, both zinc white and titanium white have been reported to catalyze the formation of hydrogen peroxide from oxygen and water which are small molecules present in some quantity in any paint film.19 The peroxide is a strong oxidant capable of destroying many types of chromophores. Since some paint bleaching also occurs in the absence of oxygen, photochemical pathways other than photooxidations must also play a role in the color recovery process. The relative efficiencies with which different scorched paints are bleached by ultraviolet and visible light are not the same for all paint samples suggesting that paint pigments do have an influence on the process. The influence is not one which is easily predicted as many factors can contribute. For example, certain pigments absorb more strongly in the near ultraviolet than in the visible. This would tend to reduce the rate of any bleaching which is effected by ultraviolet radiation over that caused by visible radiation. On the other hand, if the ultraviolet energy taken up by the absorbing pigment leads to the creation of a chromophore-consuming oxidant, such as singlet oxygen or hydrogen peroxide, then such absorption can cause ultraviolet light to be the more effective bleach. An important question relevant to this study concerns effects other than bleaching which bleaching radiation can have on scorched paint medium and pigments. Aside from destroying fugitive pigments, one such effect of the radiation can be that of causing the disintegration of medium molecular structure and the creation of molecular groups which, while colorless in themselves, can lead to eventual color reversion. Qualitative examination of bleached paint samples over a period of a year or more following bleaching show no evidence of post-bleach darkening. While no measurements have been made on changes in the integrity of the paint films as a function of light exposure time, no obvious alterations have been observed. Of the two light sources, however, the ultraviolet can be expected to have the more deleterious effect on a paint medium. Radiation can cause photochemical damage only if it is absorbed by a material, and when absorbed it is often most damaging to the absorbing molecular group. In a discolored medium the only chromophores which absorb visible light are those responsible for the medium color, and so they may be selectively destroyed by it. Ultraviolet radiation, however, can be absorbed both by the visible light absorbing chromophores (a chromophore absorbing in the visible range usually also absorbs in the ultraviolet) and also by certain 3 CONCLUSIONSTHE RESULTS of the present study show that radiation in both the visible and long wavelength ultraviolet ranges can effect substantial recovery of color in heat darkened oil paint films. With regard to the potential use of this bleaching method in the conservation of heat damaged paintings, the following points are pertinent:
The scope of the investigation reported here has not covered many aspects of the light bleaching process. Despite its promise additional studies will be necessary to establish how useful a tool light bleaching will become to the conservation field. The author is continuing research bearing on this matter. ACKNOWLEDGEMENTSTHE AUTHOR IS GRATEFUL for the support of this work provided by an award, in 1976, of a summer faculty research fellowship by the Research Foundation of the State University of New York. He also expresses his sincere appreciation to Caroline and Sheldon Keck for their constant encouragement and support. APPENDIX1 APPENDIXOIL PAINTS used in the experiments were all Winsor & Newton products. Information on each given here is that supplied by the manufacturer.
∗Polarized light microscopy and microchemical tests established that these paints included the pigments stated on the label. The ultramarine deep also contained a white pigment (an inert?) having a refractive index below 1.72. The cobalt blue and cadmium yellow deep may also have contained some amount of white filler and organic colorant, respectively, but this was not firmly established. REFERENCESA. G.Boisonas, “The Treatment of Fire Blistered Oil Paintings,” Studies in Conservation, 8 (1963): 55–66. T. duP.Cornelius, “Further Developments in the Treatment of Fire Blistered Oil Paintings,” Studies in Conservation, 11 (1966): 31–36. F. F.Jones and B.Rabin, “On Reclaiming Damaged Paintings,” University, A Princeton Quarterly, No. 59 (1974): p. 22–27. AKetnath, “The Treatment of a Fire-Damaged Picture Painted on Masonite Board,” Studies in Conservation, 23 (1978): p. 168–173. A. P.Laurie, The Painter's Methods and Materials, J. B. Lippincott Company, Philadelphia (1926): p. 38. M.Phillips and C.Whitney, “The Restoration of Original Paints at Otis House,” Old-Time New England, The Bulletin of the SPNEA, 62, no. 1 (1971): p. 25–28. M.Phillips, “Problems in Paint Restoration,” Preservation and Conservation: Principles and Practice, S.Timmons, ed., Smithsonian Institution Press, Washington (1976): p. 274–285. H. W.Levison, Artists Pigments. Lightfastness Tests and Ratings, COLORLAB, Hallandale, Florida (1976). The drying oil in the Winsor & Newton tube oil paints used in the study (see the Appendix) was either safflower oil or was unspecified, probably linseed oil. As all common artists' drying oils are unsaturated triglycerides with similar structures and chemistry, which ones were used in the experiments should have little influence on the results obtained. Winsor & Newton “Artists' Rectified Turpentine” and “Artists' Linseed Oil” were used. Labline Imperial II Radiant Heat Oven, Labline Instruments Incorporated, Melrose Park, Illinois, equipped with a 1650 watt auxiliary wire coil heater and small fan. General Electric Company publication TP-111, Fluorescent Lamps, Lamp Business Division, Nela Park, Cleveland, Ohio (1973): p. 8–9. Measurements obtained with a Gossen Luna-Pro sbc model exposure meter, Berkey Marketing Company, Woodside, New York. This figure was obtained using data taken from the IES Lighting Handbook, 5th ed., J. E.Kaufman and J. F.Christensen, eds., Illuminating Engineering Society, New York (1972): p. 8–109. Total reflectance spectra were also recorded. These and visual examination of the paint did show change in surface texture and reflectance occurred in some runs but these were small. For an introductory discussion of the CIE Color Systems see Principles of Color Technology, F. W.Billmeyer, Jr., and M.Saltzman, Interscience Publishers, New York (1966): esp. p. 33ff. Ref. 8: p. 24. B.Ranby and J. F.Rabek, “Photooxidative Degradation of Polymers by Singlet Oxygen,” Ch. 26 in Ultraviolet Light Induced Reactions, S. S.Labana, ed., American Chemical Society, Washington (1976): p. 391ff. G. S.Egerton, “The Role of Hydrogen Peroxide in the Photochemical Bleaching of Cotton Sensitized by Vat Dyes and Some Metallic Oxides,” Journal of the Textile Institute, 39 (1948): T305ff.
 Section Index Section Index |