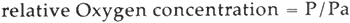THE INFLUENCE OF OXYGEN ON THE FADING OF ORGANIC COLORANTSJ. S. Arney, A. J. Jacobs, & R. Newman
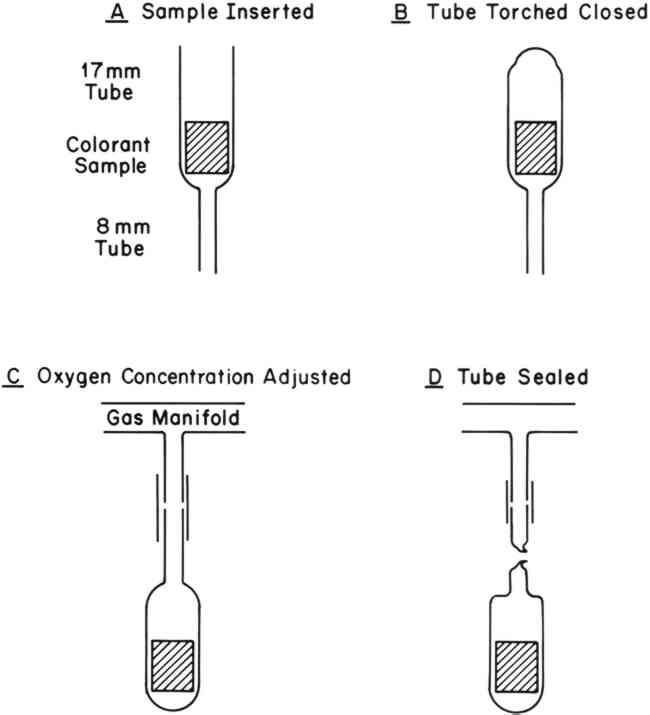
3 EXPERIMENTAL3.1 Colorant SamplesThe colorant systems listed in Table I were selected for study from those on hand in our laboratory, and all were found to be particularly fugitive. Although these colorants represent only a small sample of the colorants one might encounter in museum objects, they include a variety of molecular types. Thus, we felt they would be representative of systems that one might wish to protect with an oxygen-free environment. TABLE I COLORANT SYSTEMS EXAMINED 3.2 Sample PreparationRates of fading of the colorant systems listed in Table I were determined under various concentrations of oxygen ranging from 21% (an atmosphere of air) to 0% (nitrogen). Control of the oxygen concentration was achieved by sealing the test samples in pyrex glass tubes as shown in Figure 2. The tubes were evacuated, filled with dry air (R.H = 0%) and then evacuated to a pre-determined pressure. Dry nitrogen was then allowed to enter the tubes to bring the total pressure up to one atmosphere.
Oxygen concentrations are expressed relative to the concentration of oxygen in one atmosphere of air, as shown in equation 1,
where P and Pa are the partial pressures of oxygen in the tube and in an atmosphere of air, respectively. The lowest oxygen concentration achieved in this project was P/Pa = 0.0013. The highest oxygen concentration examined was P/Pa = 1.00. 3.3 Accelerated FadingThe sealed pyrex tubes containing the colorant samples were exposed to a bank of G.E. Daylight fluorescent tubes (F48T 12). The average intensity of light reaching the samples was 1 � 104 lux. Each sample was exposed for a known amount of time, and the total exposure for each sample, in terms of lux-hours, was calculated. Under the experimental conditions, the total light striking the samples undoubtedly included some ultraviolet radiation above 280 nm. No attempt was made to differentiate the influence of ultraviolet and visible light. 3.4 Reflectance MeasurementsReflectance measurements were made on the colorant samples before and after exposure to light. A Kollmorgen model D-1 “Color-Eye” reflectance spectrophotometer, modified for small area viewing, was used. Measurements were made relative to barium sulfate at the wavelength shown in Table I. The specular component of the reflected light was excluded in the measurement. 3.5 Determination of Fading RatesRates of fading were expressed as relative rate constants, kr', defined in Equation 2.
Values of kr were obtained experimentally by dividing the time required for a colorant sample to fade an arbitrarily chosen amount by the time required to fade the same amount at the chosen oxygen concentration. Times were expressed in terms of lux-hours of exposure. Full discussions of this time-ratio method of kinetic analysis can be found in the references.10,11 The advantage of expressing the rate of fading in terms of kr and the oxygen concentration in terms of P/Pa is that the influence of oxygen concentration on the fading of all of the colorants can be compared directly regardless of the lightfastness 3.6 Reporting of DataFrom data obtained on kr versus P/Pa, appropriate statistical regressions were performed to determine the value of kr at P/Pa = 0 (zero oxygen concentration). Values of kr at P/Pa = 0 are listed as i in Table II. Error limits are the 95% confidence intervals obtained from the regression calculations. Correlation coefficients, r2, are also reported. In addition, the inverse, 1/i, is given in Table II. The value of 1/i is an estimate of the factor by which the life expectancy of the colorant in air would be multiplied if placed in an oxygen-free atmosphere. TABLE II SUMMARY OF DATA ON THE INFLUENCE OF OXYGEN CONCENTRATION ON THE FADING RATE OF COLORANTS |