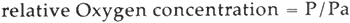THE INFLUENCE OF OXYGEN ON THE FADING OF ORGANIC COLORANTSJ. S. Arney, A. J. Jacobs, & R. Newman
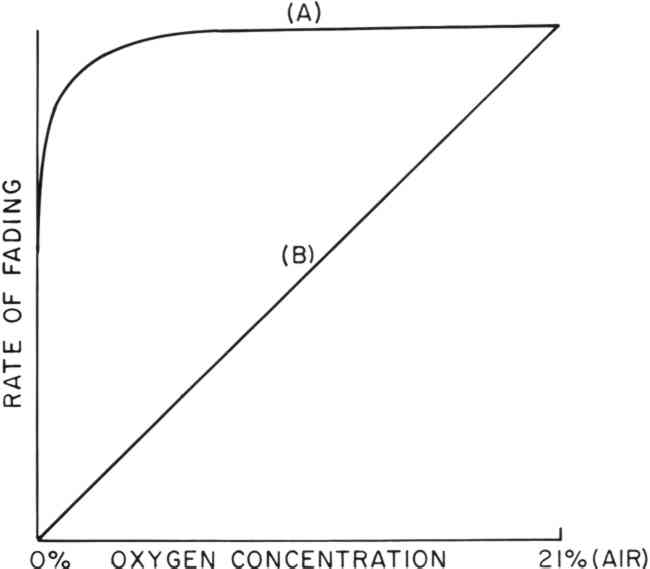
ABSTRACT—The feasibility of using an oxygen-free environment to retard the rate of fading of colorants has been explored. The factors by which the life expectancy of a variety of colorants would be multiplied in such an environment were determined by accelerated fading experiments. In general, the life expectancy of most colorants can be increased by such techniques, but a complete halt of the fading process has not been generally observed. In addition, some colorants were found to fade more quickly in an oxygen-free environment. However, it has also been found that at least 90% of the beneficial influence of an oxygen-free environment can be realized with as much as 0.2% oxygen (1% air) remaining in the environment of the colorant. Such an environment might be achieved and maintained at relatively little expense. 1 INTRODUCTIONTHAT OXYGEN in our atmosphere can cause the deterioration of many organic materials found in museum objects has long been recognized1, and the general nature of these oxidative processes has been reviewed by Feller.2,3 The possibility of decreasing the rate of fading of organic colorants in museum objects by placing the objects in an oxygen-free environment has, from time to time, been suggested, and recent work by scientists in the conservation profession has made possible the construction of “microenvironmental” cases capable of maintaining an atmosphere of an inert gas with as low as 0.06% residual oxygen.4,5 Nevertheless, the use of such cases for the storage or display of museum objects has not been widespread. This is so in part because of the expense involved in the construction and maintenance of such cases and in part because of the absence of quantitative information to indicate the degree of enhancement in the life expectancy of colorants under an inert gas environment. The objectives of this project were to determine (1) how effective an oxygen-free environment is in decreasing fading rates of organic colorants and (2) how much oxygen must be removed from a display case before a significant decrease in the rate of fading is achieved. The results indicate that the fading of many organic colorants is not halted entirely by removal of oxygen and that the degree of enhancement of life expectancy varies from colorant to colorant. On the other hand, the results also suggest that for most organic colorants at least 90% of the beneficial influence of a completely oxygen-free environment can be achieved in a display case containing as much as 0.2% residual oxygen. 2 BACKGROUNDIT HAS LONG been known that the light-induced fading of many organic colorants can be inhibited by replacing the air surrounding the colorant with an oxygen-free gas.1,6 However, a complete halt of the fading process under such conditions is generally not observed. In accounting for this non-zero rate of fading, it might seem reasonable to assume that chemical reactions are taking place that do not require oxygen. However, it is not possible to eliminate completely all of the oxygen from a Thomson has suggested that the oxidative degradation of most organic materials would show little or no decrease in rate of degradation until parts-per-million levels of oxygen were achieved.7Figure 1, curve A, illustrates the relationship between fading rate and oxygen concentration that might be observed if Thomson's prediction were to apply.
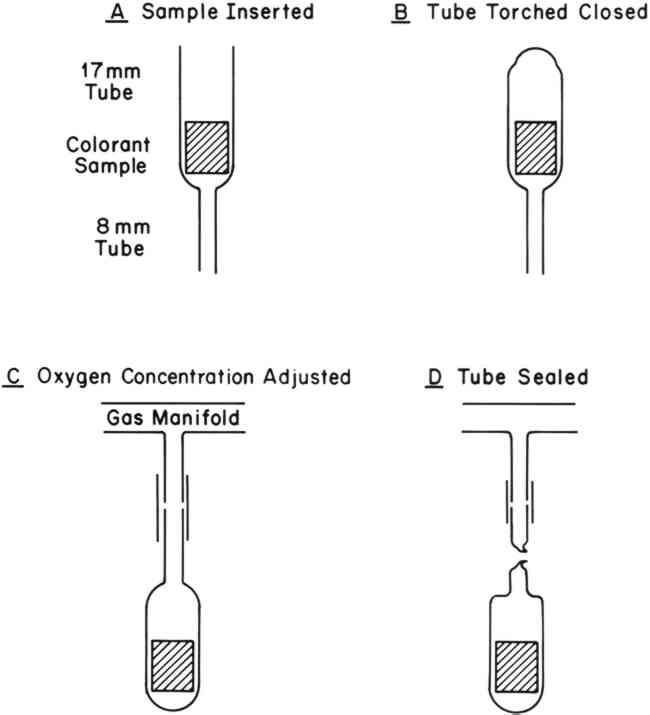
However, Giles has suggested that the rate of fading of most organic colorants should decrease linearly with a decrease in oxygen concentration8, as shown in Figure 1, curve B. If curve B were to apply, then fading could be decreased by 90% with as much as 2% residual oxygen surrounding the colorant. On the other hand, if curve A were to apply, then extremely rigorous measures would be required to achieve a significant enhancement in the permanence of a colorant. Although display cases capable of maintaining such an environment have been constructed4, the cost of construction and maintenance are prohibitive. We have found only one report, published by Lasareff in 19129, quantitatively relating rates of fading to oxygen concentration. Lasareff reported that the fading rates of two cyanine dyes decreased linearly (curve B) with a decrease in oxygen concentration. Our research was undertaken to obtain additional data on rate versus oxygen-concentration relationships. 3 EXPERIMENTAL3.1 Colorant SamplesThe colorant systems listed in Table I were selected for study from those on hand in our laboratory, and all were found to be particularly fugitive. Although these colorants represent only a small sample of the colorants one might encounter in museum objects, they include a variety of molecular types. Thus, we felt they would be representative of systems that one might wish to protect with an oxygen-free environment. TABLE I COLORANT SYSTEMS EXAMINED 3.2 Sample PreparationRates of fading of the colorant systems listed in Table I were determined under various concentrations of oxygen ranging from 21% (an atmosphere of air) to 0% (nitrogen). Control of the oxygen concentration was achieved by sealing the test samples in pyrex glass tubes as shown in Figure 2. The tubes were evacuated, filled with dry air (R.H = 0%) and then evacuated to a pre-determined pressure. Dry nitrogen was then allowed to enter the tubes to bring the total pressure up to one atmosphere.
Oxygen concentrations are expressed relative to the concentration of oxygen in one atmosphere of air, as shown in equation 1,
where P and Pa are the partial pressures of oxygen in the tube and in an atmosphere of air, respectively. The lowest oxygen concentration achieved in this project was P/Pa = 0.0013. The highest oxygen concentration examined was P/Pa = 1.00. 3.3 Accelerated FadingThe sealed pyrex tubes containing the colorant samples were exposed to a bank of G.E. Daylight fluorescent tubes (F48T 12). The average intensity of light reaching the samples was 1 � 104 lux. Each sample was exposed for a known amount of time, and the total exposure for each sample, in terms of lux-hours, was calculated. Under the experimental conditions, the total light striking the samples undoubtedly included some ultraviolet radiation above 280 nm. No attempt was made to differentiate the influence of ultraviolet and visible light. 3.4 Reflectance MeasurementsReflectance measurements were made on the colorant samples before and after exposure to light. A Kollmorgen model D-1 “Color-Eye” reflectance spectrophotometer, modified for small area viewing, was used. Measurements were made relative to barium sulfate at the wavelength shown in Table I. The specular component of the reflected light was excluded in the measurement. 3.5 Determination of Fading RatesRates of fading were expressed as relative rate constants, kr', defined in Equation 2.
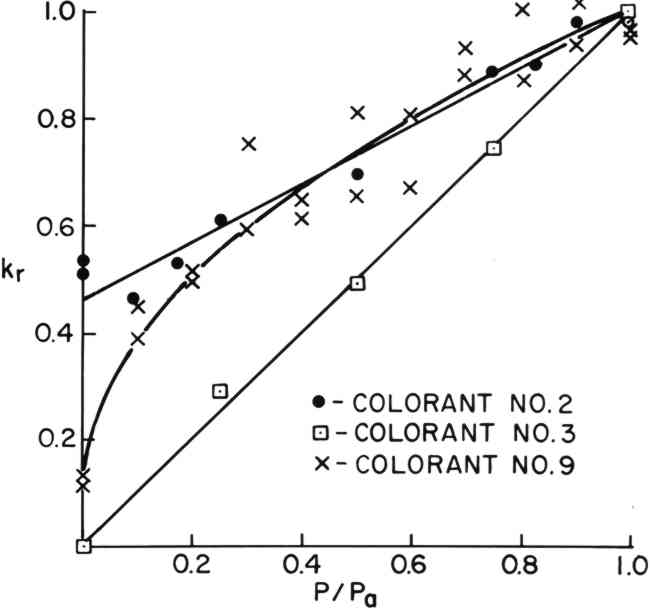
Values of kr were obtained experimentally by dividing the time required for a colorant sample to fade an arbitrarily chosen amount by the time required to fade the same amount at the chosen oxygen concentration. Times were expressed in terms of lux-hours of exposure. Full discussions of this time-ratio method of kinetic analysis can be found in the references.10,11 The advantage of expressing the rate of fading in terms of kr and the oxygen concentration in terms of P/Pa is that the influence of oxygen concentration on the fading of all of the colorants can be compared directly regardless of the lightfastness 3.6 Reporting of DataFrom data obtained on kr versus P/Pa, appropriate statistical regressions were performed to determine the value of kr at P/Pa = 0 (zero oxygen concentration). Values of kr at P/Pa = 0 are listed as i in Table II. Error limits are the 95% confidence intervals obtained from the regression calculations. Correlation coefficients, r2, are also reported. In addition, the inverse, 1/i, is given in Table II. The value of 1/i is an estimate of the factor by which the life expectancy of the colorant in air would be multiplied if placed in an oxygen-free atmosphere. TABLE II SUMMARY OF DATA ON THE INFLUENCE OF OXYGEN CONCENTRATION ON THE FADING RATE OF COLORANTS 4 RESULTSVALUES of kr were determined for the colorants listed in Table I under atmospheres with relative oxygen concentrations, P/Pa, ranging from 1.00 to 0.0013. The results of these experiments fell roughly into four experimental categories, as discussed below. 4.1 Linear Rate Versus Oxygen ConcentrationColorants #1 through #4 were found to fade at rates that varied linearly with oxygen concentration. The data for colorants #2 and #3, shown in Figure 3, are typical examples. A linear regression of kr versus (P/Pa) allowed an estimate of the rate of fading in the absence of oxygen, and these rates are shown in Table II (A) as i. Colorant #2 appears to fade significantly (i > 0) in the absence of oxygen.
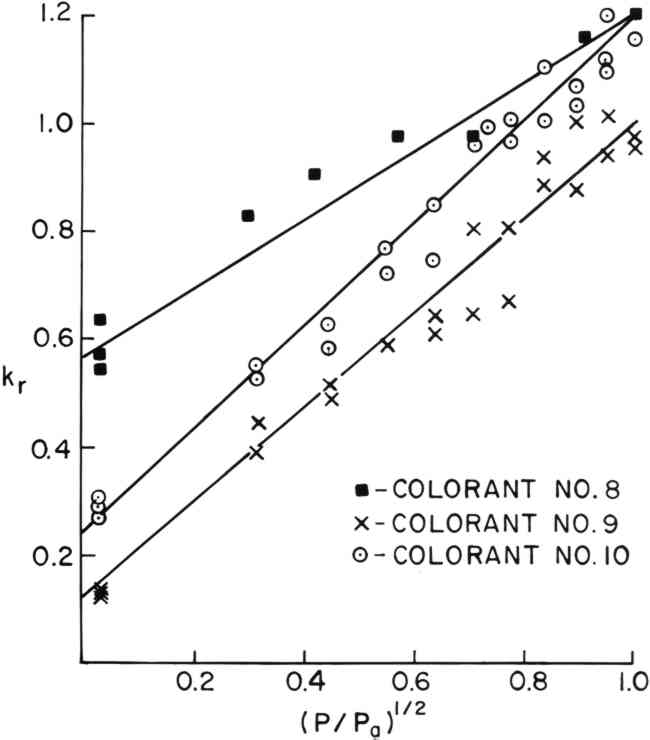
4.2 Linear Rate Versus Square Root of Oxygen ConcentrationThe rates of fading of colorants #5 through #11 did not decrease linearly with oxygen concentration but appeared to turn down sharply at the lower oxygen concentrations. Colorant #9, shown in Figure 3, is a typical example. It was found, empirically, that the rate of fading of colorants #5 through 11 varied linearly with the square root of the oxygen concentration, as shown in Figure 4 for colorants #8, #9, and #10. A linear regression of kr versus (P/Pa)� allowed an estimate of the rate of fading of these colorants in the absence of oxygen, and these rates are shown in Table II (B) as i. Colorants #5 through #8 appear to fade significantly (i > 0) in the absence of oxygen.
4.3 Experimentally Uncertain DataColorants #12 through #15 were found to fade less rapidly in the absence of oxygen, but the values of kr obtained were not of sufficient precision to allow an interpretation of the relationship between kr and (P/Pa). Linear regressions of the data with respect to both (P/Pa) and (P/Pa)� were performed. The values of i and the 95% confidence intervals for i were roughly the same for both regressions. The values of i given in Table II (C) represent the values obtained from the regression that gave the highest correlation, r2. 4.4 Rate Increase with a Decrease in Oxygen ConcentrationColorants #16 and #17 were found to fade more quickly at P/Pa = 0.0013 than at P/Pa = 1.00. Such behavior has been observed for a number of colorant systems12,13,14 and extensive studies of these two colorants were not carried out. 5 DISCUSSION AND CONCLUSIONSTHE LINEAR RELATIONSHIP between fading rate and oxygen concentration observed for colorants #1 through #4 is in accord with the prediction of Giles8 and the observations reported by Lasareff.9 However, a nonlinear relationship between kr and P/Pa was found to be the most common pattern of behavior. The down-turn in rate at lower oxygen concentrations, though less pronounced than expected, is nonetheless The linear and square root relationships indicate that the beneficial influence of an oxygen-free environment can be obtained without the need to reduce the oxygen concentration to the parts-per-million level suggested by Thomson.7 As much as 0.2% oxygen (equivalent to P/Pa = 0.01) would appear to give at least 90% of the effectiveness of a completely oxygen-free environment. Furthermore, a 0.2% oxygen concentration might be achieved easily in a practical situation at relatively little expense.17 From the data in Table II, one can estimate the practical benefit of an oxygen-free environment. The inverse of the relative rate at P/Pa = 0, (1/i in Table II) is the factor by which the life expectancy of the colorant would be multiplied if displayed in the absence of oxygen. In general, a significant enhancement in life expectancy (1/i > 10) is not observed. Furthermore, colorants #16 and #17, as well as many others reported in the literature12,13,14 fade more rapidly in the absence of oxygen. Thus, although an inexpensive display case might be constructed that is capable of achieving better than 90% of the efficiency of a truly oxygen-free environment, such a display case would be useful only if the colorants in an object have been identified, are known to be particularly fugitive, and are known to benefit significantly from an oxygen-free environment. ACKNOWLEDGEMENTSTHIS INVESTIGATION was made possible by grants from the Andrew W. Mellon Foundation and the National Endowment for the Arts. The authors also express their thanks to Dr. R. L. Feller, Director of the Center on the Materials of the Artist and Conservator, for invaluable consultation and discussion. REFERENCESAmong the earliest investigations were those conducted by M. E.Chevreul, “Recherches chimiques sur la Teinture,” M�m. de l' Acad. Roy. des Sci. de I'Inst. de France, 16, 53 (1837) and by Russell and Abney, whose work was reviewed byN.Brommell, Studies in Conservation, 9, 140 (1964). R. L.Feller, Bull. Am. Inst. Conserv., 14, 142 (1974). R. L.Feller, “Stages in the Deterioration of Organic Materials”, Advances in Chem. Series No., 164, p. 314 (1977). U.S. National Bureau of Standards Circular 505 Preservation of the Declaration of Independence and the Constitution of the United States, issued 2 July 1951. H.K�hn, London Conference on Museum Climatology, IIC, p. 79 (1967). N. S.Brommelle, Studies in Conservation, 9, 140 (1964). G.Thomson, The Museum Environment (Butterworth & Co., London and Boston: 1978), 187. C. H.Giles, “The Fading of Coloring Matters”, J. Appl. Chem., 15, 541 (1965). P.Lasareff, Z. Phys. Chem., 78, 657 (1912). A.Frank, Chemiker Ztg. Chem. Appar., 86, 174 (1962). R.Sizmann, and A.Frank, Chemiker Ztg. Chem. Appar., 87, 347 (1963). T.Padfield, and S.Landi, Studies in Conservation, 11, 181 (1966). C. H.Giles, J. W.Cumming, and A. E.McEachran, J. Soc. Dyers Colorists, 72, 373 (1956). H. C. A.vanBeek and P. M.Heertjes, “Fading by Light of Organic Dyes on Textiles and Other Materials”, Studies in Conservation, 11, 123 (1966). G.Scott, Atmospheric Oxidation and Antioxidants (Elsever Publishing Co., New York: 1965), 99–114, 424. J. R.Shelton and W. L.Cox, Ind. Eng. Chem., 46, 816 (1954). For a thorough review of techniques for establishing inert gas environments, see D. F.Shriver, The Manipulation of Air Sensitive Compounds (McGraw-Hill, New York: 1969), 164–189.
 Section Index Section Index |