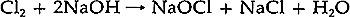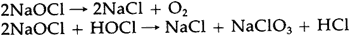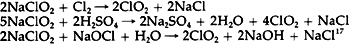TREATMENT OF A SALT IMPREGNATED WOODCUT BY E.L. KIRCHNERWeston Craigen
2 DETERMINATION OF THE CAUSE OF DETERIORATIONIT WAS DECIDED that a small portion of the margin should be sacrificed to provide samples for testing to identify the materials present and, later, to be used as small-scale models of different methods for treating the print. The generous size of Examination of fibers from the paper under 10–20x magnification with the polarizing microscope and comparison with standard fiber samples revealed that the sheet is made primarily from wood pulp.2 The short length of the fibers and their lack of typical diagnostic features due to their damaged state prevented a more specific identification. Staining of the fibers with “C” stain was attempted to determine the type of wood from which the paper was made and its method of manufacture.3 The results were inconclusive because the fibers remained uncolored. The crystalline appearance of the unknown material which permeated the sheet suggested that X-ray diffractometry might be a suitable analytical method to determine its structure and composition.4 In preparing the sample for the diffractometer, it was not initially considered necessary to separate the crystalline material from the paper fibers as it was thought that the cellulose probably would not exhibit an X-ray diffraction pattern intense enough to interfere with the analysis. From visual examination, the unknown crystals seemed to be distributed uniformly throughout the paper, and so the sample was not ground up and spread evenly on a glass slide as in the usual manner of sample preparation. Instead, the sample, a piece of the margin of the print measuring approximately 1.5 cm square, was mounted on a slide with pressure-sensitive tape.5 The mounted sample was placed in the diffractometer, and a powder diffraction pattern was obtained.6 A tentative identification was made by comparison of this pattern to standard patterns of known materials.7 A sodium chloride pattern would account for all but two of the lines. In order to determine whether the unidentified lines present in the diffraction pattern were due to the cellulose or to other crystalline materials in the paper, a further diffraction experiment was conducted. The sample was removed from the slide and ground in a mortar. It was then soaked in deionized water and heated until it was lukewarm to facilitate dissolution of any soluble salts. The insoluble matter was allowed to settle to the bottom of the beaker and the liquid was decanted into a second beaker. The second beaker was allowed to stand for several days until the water had evaporated, leaving a solute composed of discrete crystals. These crystals exhibited cubic crystal habits under 10–20x magnification. As insufficient crystals were available to obtain a satisfactory analysis using the diffractometer technique previously described, the Debye-Scherrer method of producing an X-ray diffraction pattern photographically was used.8 This technique produces identifiable patterns using much smaller samples. However, the sample must be irradiated for a longer time (17� hrs instead of � hr with this particular sample). The sample was prepared for analysis by grinding approximately 10 crystals in a mortar. Each crystal measured about 460μm on a side. The resulting powder was placed in a cupped slide and a drop of 70% collodion in amyl acetate was added. After the liquid was added it was allowed to stand for 20 min. forming a film which contained the sample. The film was peeled away from the slide, adhered to the end of a glass rod (.1 mm in diameter) with Aroclor 1260, and suspended over warm amyl acetate to shrink the film into a ball. The rod was mounted in the Debye-Scherrer camera and irradiated.9 Determination of the index of refraction of the crystals confirmed their identification. This was accomplished by comparing the index of refraction of the An elemental analysis of a sample of the Junkernboden paper was carried out with the emission spectrograph.11 The sample was prepared by grinding a piece of the paper .5 cm square in a mortar and combining the resulting powder with graphite in a graphite electrode. The spectrum of the Kirchner paper displayed strong lines characteristic of sodium, calcium, and aluminum and moderate lines of magnesium, manganese, iron, silicone, and copper. The analyses described above verify the presence of sodium chloride in the print. No method of determining the precise amount of salt in the sheet was available, but evidence obtained with the X-ray diffractometer showed that more than a “trace amount” was present.12 The origin of the sodium chloride is not known, but several hypotheses have been proposed. The most obvious is that the sheet was immersed in sea water, but it is not known whether a sufficient amount could be absorbed by this means. The emission spectrographic data showed that four of the five metallic ions usually present in sea water are present in the sheet; these are sodium, magnesium, calcium, and iron.13 Potassium is missing. Other potential causes of the salt accumulation may depend on the materials used in the paper's manufacture. The paper could have been treated or coated in such a way as to cause a deteriorating effect with its environment. An unusual sizing process might have resulted in some reaction producing salt. However, examination with the polarizing microscope supplied no information to support these hypotheses. In addition, there are no references in the literature known to the author to such processes being responsible for the presence of sodium chloride in paper. Several bleaching processes are known to produce sodium chloride. The simplest and most accessible process involves the use of sodium hypochlorite, NaOCI. This is the active ingredient in common commercial bleaches such as “Chlorox”. Sodium hypochlorite is manufactured by the process:
An equal amount of sodium chloride is produced, and generally is not removed from commercial solutions. Since sodium hypochlorite is stable only under alkaline conditions, commercial solutions are usually manufactured with a high pH to give them longer shelf lives.14 The surface pH of Junkernboden was found to be 8.15 This slight alkalinity could have been due to the high pH of such a bleach. The decomposition of sodium hypochlorite occurs in two ways, both of which result in the production of salt:
The less purified the bleach, the more the decomposition will follow the first route.16 Sodium chloride is also produced in the chlorine dioxide bleaching process. The following equations show three methods by which chlorine dioxide can be produced from sodium chlorite. All three result in the production of sodium chloride as well.
Sodium hypochlorite is more readily available than chlorine dioxide, which is both complicated to use and dangerous because of its explosive properties. In order to deposit salt in the print by either method, the print would have had to have been left unrinsed. The author has no conclusive evidence to support any of the three hypotheses concerning the origin of the salt. The idea that the inherent qualities of the materials could cause the production of sodium chloride is unsubstantiated and will be excluded from further consideration in this discussion. The presence of the appropriate matallic ions (with the exception of potassium) supports the theory that the print was immersed in sea water, while the stark whiteness and alkalinity before treatment of the paper support the bleaching hypothesis. The short lengths of the paper fibers and the lack of their usual identifying characteristics, as seen with the polarizing microscope, could be the result of over-bleaching or bleaching without rinsing. Although the evidence discussed here lends plausibility to the hypotheses that the print was immersed in sea water or bleached, a definite origin of the sodium chloride cannot be determined. A knowledge of the provenance of the print would be helpful in determining how it might have acquired the sodium chloride and suffered such damage, but, unfortunately, nothing is known of its past. It has been in the Fogg Art Museum for at least sixteen years, and its condition has not changed appreciably in that time, at least to visual examination. It was assumed that the sodium chloride had caused mechanical damage to Junkernboden by rupturing the fibers during the recrystallization process which had occurred following the changes in the relative humidity of its environment. The possibility that chemical reaction could occur between the sodium chloride and the cellulose of the paper was not studied in detail, although it is possible that such a reaction could have been detrimental to the condition of the paper. The degree to which recrystallization could have caused mechanical damage is not known to the author. However, it is certain that recrystallization at least partially caused the deterioration since “craters” had formed in the paper around individual crystals. |