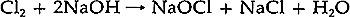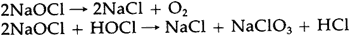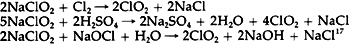TREATMENT OF A SALT IMPREGNATED WOODCUT BY E.L. KIRCHNERWeston Craigen
ABSTRACT—This paper describes the examination and subsequent treatment of Junkernboden, a woodcut printed in black and executed by E.L. Kirchner. The extremely deteriorated paper was permeated with a crystalline substance. The first phase of the project was to determine the composition of the crystalline material and the cause of the general deterioration of the paper. Use of the analytical methods X-ray diffractometry, polarizing microscopy, and emission spectrography yielded the information that sodium chloride crystals were present throughout the sheet. Several theories of their origin are discussed.The second aspect of the project was to devise a conservation treatment which would return the print to exhibitable condition. The method selected was to extract the sodium chloride crystals with wet blotters and then to line the weakened paper with Japanese tissue. The print was matted in such a way as to provide the sheet with additional support. It is now stored with other Kirchner prints in the Print Department of the Fogg Art Museum. 1 INTRODUCTIONTHIS PAPER describes the conservation of an impression of Junkernboden by Ernst Ludwig Kirchner, executed in 1918 and now in the collection of the Fogg Art Museum. Junkernboden is a woodcut printed in black ink; it depicts a scene in the Swiss Alps. When first examined by the author, the print was in such a weak condition that it could be stored only in a horizontal position, resting on a rigid support (Fig. 1). It was impossible to handle the sheet directly; any attempt to lift the unsupported print caused the paper to break into small fragments. Turning it over required that it be sandwiched between two stiff supports and then inverted.
The surface apperance of the paper was granular and generally white. Visual examination with a binocular microscope at 10x magnification showed that the paper surface was extremely irregular, with a topography of “mountains” and “valleys”.1 Small holes gave the sheet a “pock-marked” appearance visible to the naked eye, and the paper was covered with a coarse crystalline substance. A finger rubbed gently across the sheet would dislodge this substance together with paper fibers. In short, the print was virtually untouchable and completely unexhibitable. There was a brown stain on the print, localized in the lower left corner. The margins of the verso exhibited brown residues of adhesive from pressure-sensitive tapes. Also, brown staining corresponding to the inked areas of the image, probably related to the oil in the ink, was visible on the verso. None of these local disfigurements seemed to be the cause of the print's damaged state. Before actual treatment could be undertaken two problems had to be solved: 1) to discover the cause of the deterioration, which was believed to be related to the unknown crystalline material permeating the sheet, and 2) to devise a treatment that would return the print to exhibitable condition. It must be emphasized that it was by no means certain that the print could ever be made structurally secure. 2 DETERMINATION OF THE CAUSE OF DETERIORATIONIT WAS DECIDED that a small portion of the margin should be sacrificed to provide samples for testing to identify the materials present and, later, to be used as small-scale models of different methods for treating the print. The generous size of Examination of fibers from the paper under 10–20x magnification with the polarizing microscope and comparison with standard fiber samples revealed that the sheet is made primarily from wood pulp.2 The short length of the fibers and their lack of typical diagnostic features due to their damaged state prevented a more specific identification. Staining of the fibers with “C” stain was attempted to determine the type of wood from which the paper was made and its method of manufacture.3 The results were inconclusive because the fibers remained uncolored. The crystalline appearance of the unknown material which permeated the sheet suggested that X-ray diffractometry might be a suitable analytical method to determine its structure and composition.4 In preparing the sample for the diffractometer, it was not initially considered necessary to separate the crystalline material from the paper fibers as it was thought that the cellulose probably would not exhibit an X-ray diffraction pattern intense enough to interfere with the analysis. From visual examination, the unknown crystals seemed to be distributed uniformly throughout the paper, and so the sample was not ground up and spread evenly on a glass slide as in the usual manner of sample preparation. Instead, the sample, a piece of the margin of the print measuring approximately 1.5 cm square, was mounted on a slide with pressure-sensitive tape.5 The mounted sample was placed in the diffractometer, and a powder diffraction pattern was obtained.6 A tentative identification was made by comparison of this pattern to standard patterns of known materials.7 A sodium chloride pattern would account for all but two of the lines. In order to determine whether the unidentified lines present in the diffraction pattern were due to the cellulose or to other crystalline materials in the paper, a further diffraction experiment was conducted. The sample was removed from the slide and ground in a mortar. It was then soaked in deionized water and heated until it was lukewarm to facilitate dissolution of any soluble salts. The insoluble matter was allowed to settle to the bottom of the beaker and the liquid was decanted into a second beaker. The second beaker was allowed to stand for several days until the water had evaporated, leaving a solute composed of discrete crystals. These crystals exhibited cubic crystal habits under 10–20x magnification. As insufficient crystals were available to obtain a satisfactory analysis using the diffractometer technique previously described, the Debye-Scherrer method of producing an X-ray diffraction pattern photographically was used.8 This technique produces identifiable patterns using much smaller samples. However, the sample must be irradiated for a longer time (17� hrs instead of � hr with this particular sample). The sample was prepared for analysis by grinding approximately 10 crystals in a mortar. Each crystal measured about 460μm on a side. The resulting powder was placed in a cupped slide and a drop of 70% collodion in amyl acetate was added. After the liquid was added it was allowed to stand for 20 min. forming a film which contained the sample. The film was peeled away from the slide, adhered to the end of a glass rod (.1 mm in diameter) with Aroclor 1260, and suspended over warm amyl acetate to shrink the film into a ball. The rod was mounted in the Debye-Scherrer camera and irradiated.9 Determination of the index of refraction of the crystals confirmed their identification. This was accomplished by comparing the index of refraction of the An elemental analysis of a sample of the Junkernboden paper was carried out with the emission spectrograph.11 The sample was prepared by grinding a piece of the paper .5 cm square in a mortar and combining the resulting powder with graphite in a graphite electrode. The spectrum of the Kirchner paper displayed strong lines characteristic of sodium, calcium, and aluminum and moderate lines of magnesium, manganese, iron, silicone, and copper. The analyses described above verify the presence of sodium chloride in the print. No method of determining the precise amount of salt in the sheet was available, but evidence obtained with the X-ray diffractometer showed that more than a “trace amount” was present.12 The origin of the sodium chloride is not known, but several hypotheses have been proposed. The most obvious is that the sheet was immersed in sea water, but it is not known whether a sufficient amount could be absorbed by this means. The emission spectrographic data showed that four of the five metallic ions usually present in sea water are present in the sheet; these are sodium, magnesium, calcium, and iron.13 Potassium is missing. Other potential causes of the salt accumulation may depend on the materials used in the paper's manufacture. The paper could have been treated or coated in such a way as to cause a deteriorating effect with its environment. An unusual sizing process might have resulted in some reaction producing salt. However, examination with the polarizing microscope supplied no information to support these hypotheses. In addition, there are no references in the literature known to the author to such processes being responsible for the presence of sodium chloride in paper. Several bleaching processes are known to produce sodium chloride. The simplest and most accessible process involves the use of sodium hypochlorite, NaOCI. This is the active ingredient in common commercial bleaches such as “Chlorox”. Sodium hypochlorite is manufactured by the process:
An equal amount of sodium chloride is produced, and generally is not removed from commercial solutions. Since sodium hypochlorite is stable only under alkaline conditions, commercial solutions are usually manufactured with a high pH to give them longer shelf lives.14 The surface pH of Junkernboden was found to be 8.15 This slight alkalinity could have been due to the high pH of such a bleach. The decomposition of sodium hypochlorite occurs in two ways, both of which result in the production of salt:
The less purified the bleach, the more the decomposition will follow the first route.16 Sodium chloride is also produced in the chlorine dioxide bleaching process. The following equations show three methods by which chlorine dioxide can be produced from sodium chlorite. All three result in the production of sodium chloride as well.
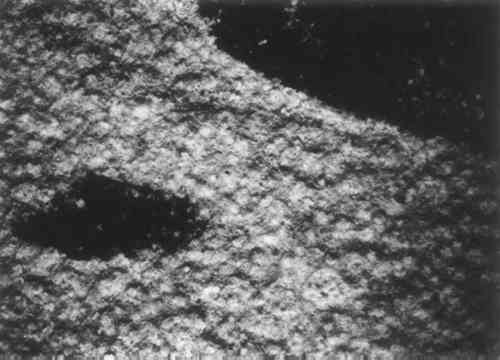
Sodium hypochlorite is more readily available than chlorine dioxide, which is both complicated to use and dangerous because of its explosive properties. In order to deposit salt in the print by either method, the print would have had to have been left unrinsed. The author has no conclusive evidence to support any of the three hypotheses concerning the origin of the salt. The idea that the inherent qualities of the materials could cause the production of sodium chloride is unsubstantiated and will be excluded from further consideration in this discussion. The presence of the appropriate matallic ions (with the exception of potassium) supports the theory that the print was immersed in sea water, while the stark whiteness and alkalinity before treatment of the paper support the bleaching hypothesis. The short lengths of the paper fibers and the lack of their usual identifying characteristics, as seen with the polarizing microscope, could be the result of over-bleaching or bleaching without rinsing. Although the evidence discussed here lends plausibility to the hypotheses that the print was immersed in sea water or bleached, a definite origin of the sodium chloride cannot be determined. A knowledge of the provenance of the print would be helpful in determining how it might have acquired the sodium chloride and suffered such damage, but, unfortunately, nothing is known of its past. It has been in the Fogg Art Museum for at least sixteen years, and its condition has not changed appreciably in that time, at least to visual examination. It was assumed that the sodium chloride had caused mechanical damage to Junkernboden by rupturing the fibers during the recrystallization process which had occurred following the changes in the relative humidity of its environment. The possibility that chemical reaction could occur between the sodium chloride and the cellulose of the paper was not studied in detail, although it is possible that such a reaction could have been detrimental to the condition of the paper. The degree to which recrystallization could have caused mechanical damage is not known to the author. However, it is certain that recrystallization at least partially caused the deterioration since “craters” had formed in the paper around individual crystals. 3 CONSERVATION OF JUNKERNBODENTHE SECOND PHASE of this project was to devise a means of removing the salt from the paper and returning the print to the healthiest possible state. Rinsing the sheet in water, an obvious way to remove sodium chloride, was precluded since the weakened paper could not sustain immersion: a small sample placed in deionized water fragmented immediately upon wetting. Therefore, it was necessary to immobolize the fibers so that they could be penetrated by water to dissolve the salt without themselves disintegrating. Several ways of doing this were considered: 1) facing the print with a porous material, 2) placing the print on a suction table, 3) consolidating it with a synthetic resin, and 4) sandwiching it between sheets of a porous material which would support the paper without adhering to the fibers. The idea of facing the print was abandoned immediately because tests proved that it would be impossible to remove the facing without displacing the surface of the print. For several reasons the use of a suction table was eliminated. At the time a suction table was not available. More importantly, the pulpy nature of the paper when wet made it certain that any irregularities or surface texture from the table would be firmly impressed in the paper. Consolidation materials were considered at length, and a test was performed using soluble nylon on a piece of the margin. This consolidant was selected as the only material customarily used in conservation that is easily permeable to water and is not also water soluble.18 Also, the matte surface produced by this resin would not be uncharacteristic of the presumed original surface of the wood-block printing ink and paper. A 2% solution of soluble nylon in ethanol was applied to the sample with a brush.19 The paper was so absorbent that it was only necessary to touch the sample in one place to saturate it. It was hoped that a consolidant would impart enough strength to the sheet to allow it to be bathed, and the soluble nylon did seem to strengthen the paper, though no quantitative tests were made to determine the degree of strengthening. However, the consolidated sample was not able to withstand the immersion in water which removal of the sodium chloride would require. The use of soluble nylon as the sole means of immobilizing the fibers during a water treatment was excluded, though the strength it lent to the sample gave promise for its use as an auxilliary treatment. Further support for the sheet, or else an alternative to immersion in water, was deemed necessary. At this point, it seemed that sandwiching the print between some porous material was the only remaining possibility for support. The sandwich arrangement would provide a method for indirect handling of the sheet but would not immobilize the fibers as, in theory, a consolidant could. Therefore, it would be necessary to remove the salt without immersing the print in water. A method employed by conservators to remove salt from pottery and stone was studied as an alternative. This procedure makes use of wet paper-pulp poultices or blotters to leach the salt from an object.20 In this case a porous interlayer surrounding the sheet would prevent loose paper fibers from adhering to the poultice or blotter. The material chosen for this purpose was a monofilament nylon woven cloth available in a variety of meshes.21 Since it is woven from single fibers (instead of multiple fibers twisted together to form a thread), it is not fuzzy and does not catch on materials with which it comes into contact. This, together with its inertness to the materials used in the project, made it suitable for use as an interlayer in treatment with poultices. For ease in description in the following paragraphs, this nylon cloth will be referred to by its trade name, Nitex. Impregnation of the damaged paper with soluble nylon was still considered a possibility for imparting strength to the print. Therefore, tests were carried out on two samples from the margin of the print to determine the advantages of the procedures under consideration. One sample was impregnated with a 2% solution of soluble nylon, dropped on the paper surface with a pipette; the other was not consolidated. When the impregnated sample had dried, both samples were sandwiched between Nitex of 195 mesh, and wet blotters were placed on top. Paper-pulp poultices were not used because they were difficult to apply uniformly and because the flat print did not require material which would mold to irregular contours. It was expected that the dissolved sodium chloride would be pulled into the blotters by capillary action as the blotters dried, and testing the blotters for chlorides after they were removed confirmed this.22 When the blotters were dry, they were removed from the samples and replaced with wet ones. Blotter changes were repeated until, after the eighth change, the chloride test yielded virtually no reaction. The Nitex was easily peeled from the surfaces of the samples, and the papers were examined. In the consolidated sample the soluble nylon had not inhibited the removal of the sodium chloride, but it had not appreciably added to the security of the paper. Since treatment with blotters The procedure finally chosen for the treatment of Junkernboden was to sandwich the print between Nitex sheets of 195 mesh and to remove the sodium chloride with wet blotters. Several factors other than those already mentioned were considered important. It was decided that the print should never be allowed to dry until as much sodium chloride as possible had been removed. Repeated recrystallization of the salt was presumed to have been responsible for at least some of the damage to the paper, so the formation of new crystals in the process of treatment was to be avoided. The use of three blotters at a time assured that the print would remain wet while the top blotter dried. A serious concern was the possibility of mold growth during the lengthy period treatment would require. To prevent this, the blotters were painted with a 7% solution of thymol in ethanol, and the ethanol was allowed to evaporate.23 The printing ink was tested for solubility in water, ethanol, and thymol in ethanol, all with no reaction. From the condition of the test samples it was known that the paper would be very weak after the sodium chloride was removed. It would be necessary to provide the sheet with additional support to prevent further damage. The traditional lining method using Japanese paper and starch paste was chosen because this method of supporting weakened paper most allows the sheet to retain its integrity. Overall mounting on a solid support would make the paper look unnaturally flat and would also prevent the sheet from expanding and contracting at its own rate. The plan was to line the print as soon as the sodium chloride was removed and before the paper had dried. In the arrangement of the print during treatment, plexiglas was used as the bottom support to allow examination of the sheet during treatment. The print, which was sandwiched between Nitex sheets, was laid face down on the plexiglas, so that after the salt had been removed the print could be lined without manipulation. The underlying Nitex interposed a “cloudy” layer but did not obscure the image. The procedure was exactly that of the test samples. After each change of blotters, which occurred twice a day, the used set was tested for chlorides. For consistency, it was decided that 1 sq. in. of each blotter would be soaked in 20 ml of deionized water for 15 min. The chloride test was performed with 10ml of the resulting solution. The extremely strong initial reaction changed to a moderate reaction by the sixth change of blotters, and to a weak reaction by the tenth. The procedure was continued through thirteen changes of blotters, by which time there was virtually no reaction at all. Ten days had elapsed since the treatment had been begun. It is interesting to note which of the three blotters absorbed the most sodium chloride. After the second change of blotters each of the blotters was tested separately; the middle one produced a considerably weaker reaction than the upper and lower ones. At the very outset of treatment an unforeseen phenomenon occurred. Within 1� hour black stains appeared in the design areas. These stains initially seemed to be bleeding printing ink. Several were streaks and others were tidal rings. All were confined to the upper half of the image, and all were associated with inked areas. None changed appreciably during the remainder of the treatment. They were not reactive to tests with thymol crystals or salt water, so their cause remains unknown. On examination after the treatment was completed and the print was dried, it was decided that they are not of the same black hue nor the same texture as the printing ink. When the salt removal was completed the Nitex was peeled from the verso. The print was lined first with a thin Japanese paper and then with a heavy one.24. The light-weight paper was used to form a good bond with the reverse of the damaged paper, while the heavier paper provided a strong support. The wheat starch paste was brushed on the lining papers and the linings were applied with hand pressure.25 The print was then turned over for the first time since treatment began and the Nitex was removed from the front. Again, an unforeseen development occurred. A pale gray counterproof of the ink image had been transferred from most areas of the design onto the Nitex. More critically, the brown stain in the lower left quadrant of the print, whose visual effect had diminished during the salt extraction process, now exhibited adhesive properties. Small portions of the surface of the paper in this area stuck to the Nitex no matter how carefully it was removed, resulting in some skinning of the sheet in the design area. When the Nitex was fully removed and dried, however, bits of detached paper and ink could be peeled from the Nitex and replaced on the print, which was still moist, with dilute wheat starch paste left over from the lining process. Virtually all of the inked areas were reattached in their original locations in this way, using a before-treatment photograph as a guide. A minimal amount of the surface ultimately was lost. Later, when the print had dried, the remaining brown stain seemed more like an adhesive layer than it had prior to treatment: it had become harder, and small areas were cracked and cupped. The lined print was allowed to air-dry completely. It buckled severely, but the drying procedure did allow the fibers to re-mesh themselves without artificial constraint. To flatten the sheet it was sprayed lightly with deionized water on the reverse until it became limp and slightly expanded. The margins of the lining were then folded over the edge of a sheet of eight-ply ragboard and glued in place with PVA emulsion.26 The ragboard mount had been counterlined with Japanese paper on the verso, which was still wet and in an expanded condition when the lined print was mounted. The ragboard bowed severely away from the print as the counterlining dried. The adhered margins of the print lining were peeled off the mount, and the procedure was repeated on another piece of eight-ply ragboard which had not been counterlined. Planar irregularities were eliminated as the print dried, but at no time was the print taut across the board. Finally, the print was matted so that it could be safely stored in the Fogg Print Department with no need for special handling. The window mat and backboard were cut from four-ply ragboard. The print on its rigid support was adhered to the backboard with acid-free photocorners and white cloth tape.27 A protective mat which would act as a separator for the window mat was cut from paper-faced expanded polystyrene board, the edges of which were sealed with pressure-sensitive tape.28 The polystyrene board was not in contact with the print itself. The rag window mat was adhered to the protective mat, which was hinged to the rag backboard. This package has the appearance of a regular matted print, but supplies the fragile work of art with greater support. It is now stored with the other Kirchner prints in the Print Department of the Fogg Art Museum. 4 THE WATERMARK ON JUNKERNBODENAFTER THE SECOND CHANGE of blotters a disturbance in the surface of the paper indicated the presence of a watermark, but the watermark itself was illegible. Later, when the print had been lined and dried it was possible to use raking and transmitted light to distinguish the words: 4.1 ASOKA REGISTEREDThis mark is located in each of the four quadrants of the sheet. The letters in “A S O K A” measure approximately 22mm in height; the letters in “REGISTERED” measure approximately 15mm in height. The width of the mark at its widest is about 170mm. Other Kirchner prints were examined and three woodcuts were found with a related mark. This related mark is not a watermark, per se, but a mark stamped in an embossed plaquette, reading: 4.2 ASOKAOne of these prints, Portrait of the Art-Dealer Ludwig Schames, exhibits not only ink qualities similar to Junkernboden, but also the same brown staining from the oil in the printing ink. The paper is thick and absorbent and buff-colored. Although Junkernboden is printed on thinner paper, it is possible that its original quality was similar to that of Schames. According to Dr. E. Kornfeld of Kornfeld und Klipstein, in Bern, Kirchner used this paper with “ASOKA” in the four quadrants of the sheet often during the winter 1917–18. It is a blotting paper which was probably made by the Sihl-Papermills in Zurich.29 5 SUMMARY AND CONCLUSIONSTHE TREATMENT DESCRIBED ABOVE greatly improved the condition and appearance of Junkernboden(Figure 2). The sheet is now cool white; its pH is 7. The paper surface does not look completely normal, as, with the crystals dissolved away, the more minute pock-marks are visible (compare Figures 3 and 4). The sheet is brittle; its surface is hard, and it has retained a barely perceptible impression of the woven Nylon visible under magnification, despite the latter's extremely fine weave.
This treatment achieved the original intention of returning Junkernboden to an exhibitable condition. The two undesirable and unforeseen effects—the black stains which appeared at the beginning of the salt-extraction process and the skinning which occurred when the Nitex was removed from the recto after lining—did not disfigure the print to an unacceptable degree. Possibly, these effects could have been avoided by treating the print face-up on a suction table. Perhaps the black staining would have been avoided if the water was drawn directly through the print. Also, the brown adhesive residue would not have been in contact with any other material so skinning would not have occurred. The disadvantages of this method are: 1) that the print would have had to have been manipulated while wet in order to be lined. When wet the sheet was literally in the pulp state, and any manipulation probably would have been prohibitively hazardous, and 2) the pressure of the suction table could have impressed the surface texture of the table or the support layer between the print and the table surface into the paper to a greater degree than did the pressure of the Nitex and blotters. The treatment performed involved no handling of Junkernboden, and was, therefore, the least dangerous alternative. The improved appearance and stabilized condition of the print, which no longer requires special handling, substantiate the conclusion that the treatment was the best available. The positive response of the print itself to the treatment further corroborates this judgement. As treatment progressed, the paper regained enough of a “memory” to exhibit an impressed watermark, which had been undetected in the original examination. After such an attempt to return this Kirchner print to exhibitable ACKNOWLEDGEMENTSMANY MEMBERS of the staff of the Center for Conservation and Technical Studies, Fogg Art Museum contributed to this project. Leon Stodulski, Eugene Farrell, Arthur Beale, and especially Marjorie B. Cohn supplied advice and encouragement for which I am very grateful. REFERENCESA Zeiss OPMI-6M Operating Microscope was used when a binocular microscope was required for examination under low magnification. All instrumentation employed in this study is located at the Center for Conservation and Technical Studies, Fogg Art Museum, Harvard University, Cambridge, MA. 02138. An Olympus POM Polarizing Microscope was used. The standard fiber samples were obtained from the Institute of Paper Chemistry, P.O. Box 1039, Appleton, WI. 54911. “C” Stain was supplied by the Institute of Paper Chemistry. For discussions of fiber identification see the following: Charles H.Carpenter, et. al., Papermaking Fibers: A Photomicrographic Atlas of Woody, Non-Woody, and Man-Made Fibers Used in Papermaking, Technical Publication No. 74, Syracuse, NY: State University College of Forestry, 1963; and John H.Graff, A Color Atlas for Fiber Identification, Appleton, WI: The Institute of Paper Chemistry, 1940. For a discussion of the principles of X-ray diffractometry see B. D.Cullity, Elements of X-Ray Diffraction, Reading, MA: Addison-Wesley Publishing Co., Inc., 1956. Scotch Brand Magic Transparent Tape, no. 810. The Diano Corp. X-Ray Diffraction Unit, no. XRD 8000, used in this research, produces monochromatic X-rays from copper radiation with a nickel filter. Joint Committee on Powder Diffraction Standards, Inorganic Index of the Powder Diffraction File. Cullity, p. 94. The Debye-Scherrer camera used has a diameter of 114.6mm. The film used was Industrial G Film, manufactured by Illford Photographic Materials. It was processed with Kodak Liquid X-Ray Developer and Kodak Rapid Fixer. The resulting film was analyzed with a Supper X-Ray Diffraction Instruments film reader. The refractive index liquids were manufactured by R. P. Cargille Laboratories, Inc. For a discussion of the principles of emission spectrography see Galen W.Ewing, Instrumental Methods of Chemical Analysis, 3rd. ed., New York: McGraw-Hill Book Co., Inc., 1960, p. 164. An attempt was made to determine the precise amount of sodium chloride in the sheet by first weighing a piece of the margin. This 1cm square sample was then washed in deionized water, allowed to dry, and re-weighed. The difference between the two weights was not an accurate measure of the loss of sodium chloride, however, because many paper fibers were also washed away due to the weakened condition of the paper. Cornelius S.Hurlbut, Jr., Minerals and Man, New York: Random House, 1970, p. 118. Kirk-Othmer, “Bleaching Agents,” Encyclopedia of Chemical Technology, 2nd. ed., New York: Interscience Publishers, Vol. 3, p. 555. A Beckman Zeromatic SS-3 pH Meter was used with an Ingold Combination Electrode, no. 6002–07.
Kirk-Othmer, p. 555. A.Shahin and OttoWachter, “Simplification of the Chlorine Dioxide Bleaching System,” Conservation of Paintings and Graphic Arts, IIC, Lisbon Conference, 1972, p. 955. E.DeWitte, “Soluble Nylon as Consolidation Agent for Stone,” Studies in Conservation, Vol. 20, February, 1975, p. 30. This soluble nylon was sold under the name “Calaton” by Frank W. Joel Laboratory Chemicals, P.O. Box 6, Downham Market. Norfolk PE38 9ED, England. H. J.Plenderleith and A. E. A.Werner, The Conservation of Antiquities and Works of Art, London: Oxford University Press, 1971, p. 304. This monofilament nylon is sold under the name “Nitex” and was obtained from Lambert Co., Inc., 920 Commonwealth Ave., Boston, MA. 02100. The test for chloride is described by Plenderleith, p. 201. The procedure here was to soak pieces of the blotters in deionized water and then test the water with 4 drops of dilute nitric acid and 5 drops of 2% silver nitrate in water. The presence of chloride ions is indicated by the formation of the white precipitate, silver chloride. Mallinkrodt, Inc. manufactured the thymol used. The thin paper used first was Tengujo; the thick paper was Kizuki-Atsuguchi. Both were obtained from Washi No Mise, RD 2, Baltimore Pike, Kennett Square, PA. 19348. The wheat starch was Aytex P and was obtained from TALAS. It was made into paste at a ratio of 1:25 with deionized water. PVA emulsion, No. R-2258, was purchased from TALAS. The ragboard was Museum Mounting Board (100% rag, acid-free) manufactured by University Products, Inc., P.O. Box 101, So. Canal Street, Holyoke, MA. 01040. The acid-free white paper used to make photocorners was obtained from Process Materials Corp., 329 Veterans Blvd., Carlstadt, N. J. 07072. Gummed cloth tape (white, nonperforated) was from Gane Bros. & Lane, Inc., P.O. Box 93843, Chicago, IL. 60670. The polystyrene board is sold under the name “Fome-Cor” and is made by the Monsanto Corp. Its edges were sealed with Scotch Brand Magic Transparent Tape, No. 810. Letter from Dr. E. Kornfeld to Mrs. Martin Cohn, December 21, 1978.
 Section Index Section Index |