THE CONSERVATION OF A PLASTIC MASK BY MARISOLW. T. Chase
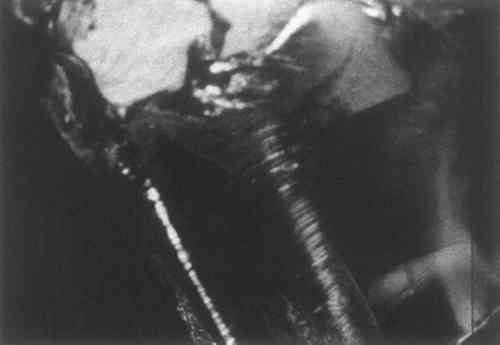
2 EXPERIMENTALFIRST, several photographs were taken of the glassy fracture in the plastic, using our Zeiss Epitechnoscope (Fig. 2). Small samples of the plastic were removed under the stereomicroscope and solubility tests were made. The plastic was not soluble in naphtha, slightly soluble in toluene, readily soluble in MI2 solvent mixture (toluene 70%, ethanol 10%, ethylene dichloride 10%, cellosolve 5%, cellosolve acetate 5%), and soluble in ethylene dichloride.
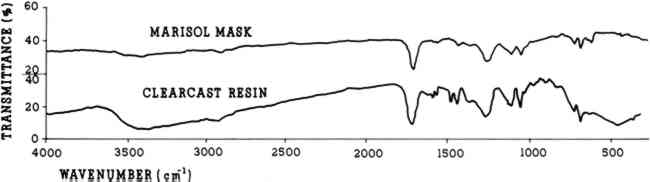
A small sample was removed from behind the proper left eye for identification by infrared spectrometry. The sample was sent to the Conservation Analytical Laboratory of the Smithsonian Institution (CAL no. 1538) and was found to be a styrenated alkyd resin (Fig. 3a). After some thought about what resins might have
|