THE EFFECTS OF WASH WATER QUALITY ON THE AGING CHARACTERISTICS OF PAPERLucia C. Tang, & Norvell M. M. Jones
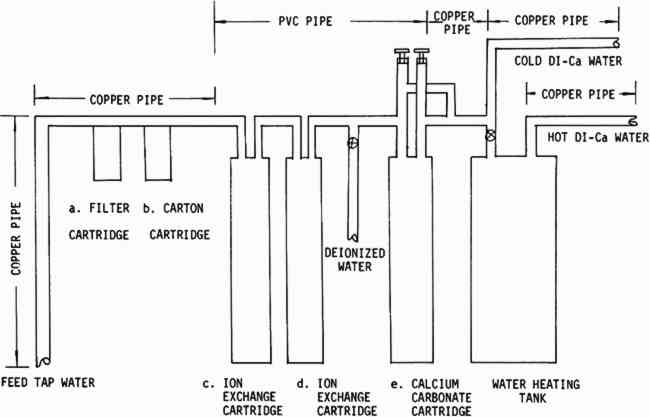
ABSTRACT—Conservators frequently wash paper to remove dirt, stains, acids, and decomposition products. Washing an acid paper with the proper water significantly increases its life. The use of high-quality water, distilled or deionized, would seem to be advisable for this purpose. However, recent work at the Library of Congress shows that such extra-pure water, containing no calcium, may shorten the life of the paper when compared with an unwashed control or Washington, D. C. municipal tap water, as measured by folding endurance and brightness tests carried out after accelerated aging in dry and humid ovens. Washington tap water has certain drawbacks because it contains chlorine which acts to oxidize cellulose, and iron and copper compounds which may act as oxidation catalysts for paper stored under humid conditions. Distilled or deionized water passed through a column of calcium carbonate chips becomes acceptable for paper washing, and can be used without shortening the life of the paper. This calcium modified water will, however, pick up copper compounds from copper pipes used to deliver it to washing sinks, and should be carried by inert piping. 1 INTRODUCTIONWATER WASHING is a widely used treatment for conservation of paper artifacts. The conservators who use this technique believe that the water usually cleans and freshens degraded papers. After washing, old papers are often brighter and new calendered papers are commonly observed to have higher folding endurance than before washing. The water seems to remove soluble acids, degradation products and some stains and to be generally beneficial to the paper. Water washing is also often used as a final step in bleaching or organic solvent treatments. Conservators have observed that tap water is unpredictable. A dry spell, or work on the water pipes may turn the water a visible rusty color. White porcelain sinks may develop suspicious green, blue, or red-brown stains under a dripping faucet. In addition to this visible evidence of contamination by iron and copper, most urban tap water contains added chlorine, occasionally strong enough to produce odor. These three contaminants are known to be harmful to cellulose,1, 2, 3, 4 and they may be present in tap water in damaging quantities. It is not surprising that conservators have sought an alternate source of water which is uncontaminated and predictable. The logic has been that if contaminated is bad, then pure must be good, and the purer the better. Many conscientious conservators have suggested that all washing, or at least the final rinse should be done in deionized or distilled water.5 At the Library of Congress, the Preservation Office began investigating a large-scale deionized system during preparation for an extensive paper treatment program. The quantity of water necessary to wash the material was too great for existing small deionizers, and the tap water at that time was very rusty. A preliminary search suggested that little testing had been done to determine the parameters of water quality acceptable for washing valuable paper items. Iron and copper in paper are known to cause degradation when the paper is artificially aged under humid conditions;3 but the critical levels of iron and copper in the water which lead to damaging pick-up by a sheet of paper during washing are not known. High purity distilled or deionized water is recognized to be aggressive, that is very reactive. This may be an advantage or a disadvantage. Aggressive wate r should be a very effective stain remover and cleaning agent, but might also strip protective ions from the cellulose and leave it vulnerable to future degradative attack. Discussions with the representative of a major water treatment company were carried out which resulted in a system which seemed to answer the Restoration Office needs and provide some interesting possibilities for exploration. This system (Figure 1) passes the water through a 25 micron filter followed by a carbon cartridge with high adsorptive capacity for organic contaminants. The carbon also removes chlorine and chloramines. The water then passes through a weakly acidic cation exchanger column followed by a weakly basic anion exchanger. The water which has passed through both columns is relatively pure (Table VI) although it is slightly acidic due to silica and carbon dioxide which are not removed. The water then enters a third column approximately the same size as the exchanger columns, filled with calcium carbonate chips, to be neutralized and to pick up calcium to replace some of that removed in the ion exchange column. The calcium carbonate column is
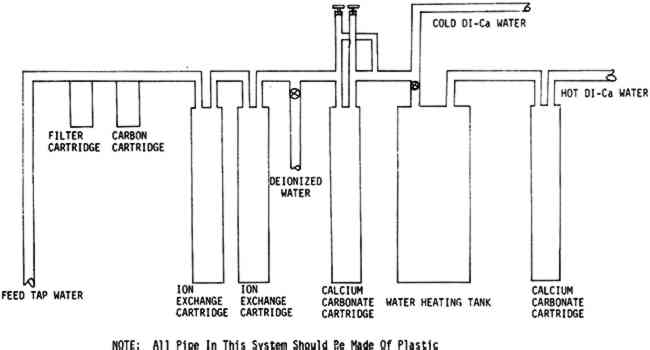
TABLE VI WATER ANALYSES FROM VARIOUS WATER SYSTEMS The design of the system was limited in part by the space available, (approximately three by six feet of floor space) and by a reluctance to replace all the existing copper plumbing until the quality of water had been fully investigated. Once the system was installed, the testing which is described in this paper was initiated to attempt to find answers to some of the questions stated above. Two modern machine-made papers were studied, as was a handmade paper from a book published in France during the late 18th Century.8 The variations in the handmade paper made it impossible to correlate the data in a meaningful way, but the trends seem to be similar to those which we observed in the modern papers. A preliminary notice of this work was published in the A. I. C. Newsletter in August, 1978. In a later issue in February, 1979, William K. Wilson of National Archives and Records Service wrote supporting our work. On the basis of test results and experience with this system, certain modifications are being made. The copper pipe will be replaced with an inert pipe to eliminate this source of possible contamination. We also plan to introduce more calcium carbonate and perhaps magnesium carbonate into the water than that obtained from the present calcium carbonate chip column. The present hot water heater is inadequate for prolonged use so more capacity for heating will be provided. One proposed revision in the system is illustrated in Figure 2.
2 EXPERIMENTAL2.1 ApparatusTHE DEIONIZED WATER was prepared in the Research and Testing Lab by passing tap water through two cation exchange cartridges (Universal Cartridge, no. 1506–20, Cole-Parmer Instrument Co., and Research Cartridge, no. 1506–30, Cole-Parmer Instrument Co.) to remove metal ions. The distilled water was prepared by distilling deionized water, using a Corning Model AG3 still. The weak base deionized water was prepared in the Restoration Workshop by the Culligan system described in the introduction (Figure 1). The cation resin was Cullex resin CH-1. The anion resin CW-2 was supplied by the Culligan Company. The calcium carbonate chips were pea-sized crushed marble also supplied by Culligan as Cullnu filter medium and described by them as containing 98.3% CaCO3, 0.5% MgCO3, and 0.03% Fe2O3, and 0.001% CuO. 2.2 Sample PreparationTHE TWO PAPERS chosen for these different waters were: 1) Champion Foldur Kraft, a bleached southern kraft paper containing rosin size and fillers from a single roll of known composition, and, 2) newsprint from cut sheets. Table I shows the paper samples selected for the tests. The five different types of water used for experimental washing of these papers were: 1) laboratory-quality deionized water—LQDI, 2) laboratory quality distilled water—distilled, 3) weakbase deionized water—WBDI, 4) weak-base deionized water passed through calcium carbonate chips—WBDI-Ca, 5) weak-base deionized water passed through calcium carbonate chips and heated to 40�C—WBDI-Ca at 40�C, and 6) Washington tap water—WT at 40�C. TABLE I PAPER SAMPLES SELECTED FOR THE TESTS Ten 8″ � 10″ pieces of Foldur Kraft and ten sheets of newsprint were used for each washing procedure. Papers were prewet with a 1:1 solution of denatured alcohol and distilled water, then put immediately into the wash water. Washing consisted of immersing the sample set of 10 sheets in each test water and leaving them immersed for either one, two or three hours. In the standard procedure the paper was held between diamond-shaped, open mesh rubber matting for support, and these “sandwiches” were stacked in a polypropylene tray. Water entered the tray from one side through a diffusion tube which distributed it equally across the tray, passed through the stack of sheets, and flowed out the lower lip of the tray at the opposite side. The sheets were at all times surrounded by moving water. The water flowed through the stack of paper at 0.4 to 1 liters/min. A variation of this procedure was used for the samples washed in WBDI water. We did not want the water to pass through the copper pipes and had no other way to get flowing water to the washing sinks. Still baths of water, drawn from the Culligan system before the calcium tank, were substituted for circulating baths in the treatments involving WBDI water. The water in these baths were changed every 10 minutes. For each paper, both Foldur Kraft and newsprint, there were 18 washed sample sets and one unwashed set. The air dried samples were subjected to accelerated aging in both humid (90�C/50% r.h.) and dry (100�C) circulating air ovens for one, two, three, and five weeks. At regular seven-day intervals one sheet from each sample set was removed and tested for folding endurance and brightness. 2.3 TestsTHE PAPER was tested to determine folding endurance, brightness, pH, and total acidity and was analyzed for iron, copper, and calcium.
3 RESULTS AND DISCUSSION3.1 Folding EndurancePAPER DEGRADATION was evaluated by measuring M. I. T. folding endurance at 1/2 kg load. Following Barrow11 and others12 the logarithm of folding endurance was taken as a linear function of days in the oven, and data was fitted to this relation by the method of least squares. The number of days for folding endurance to drop to a value of 1 was then determined by extrapolation. Life expectancy was determined as the number of days of artificial aging a paper could tolerate before folding endurance dropped to one fold. The value of treatment index was calculated as follows:
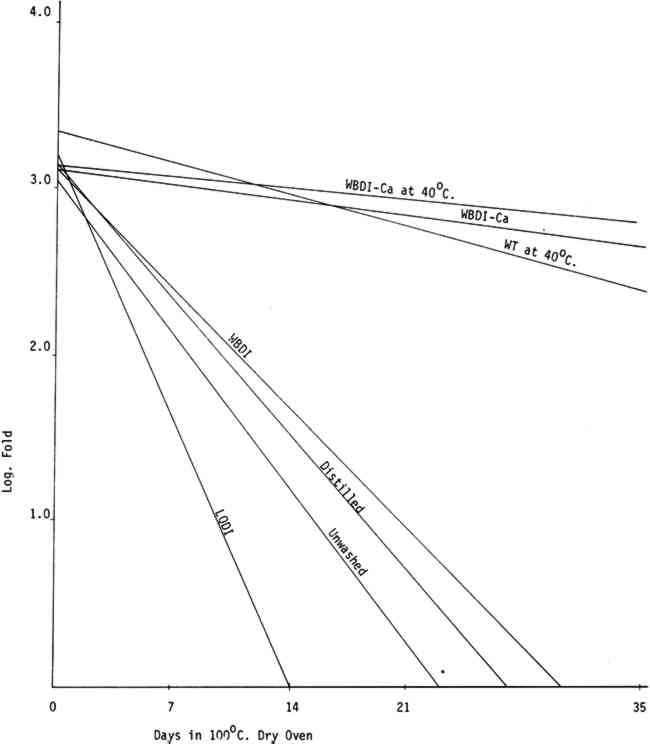
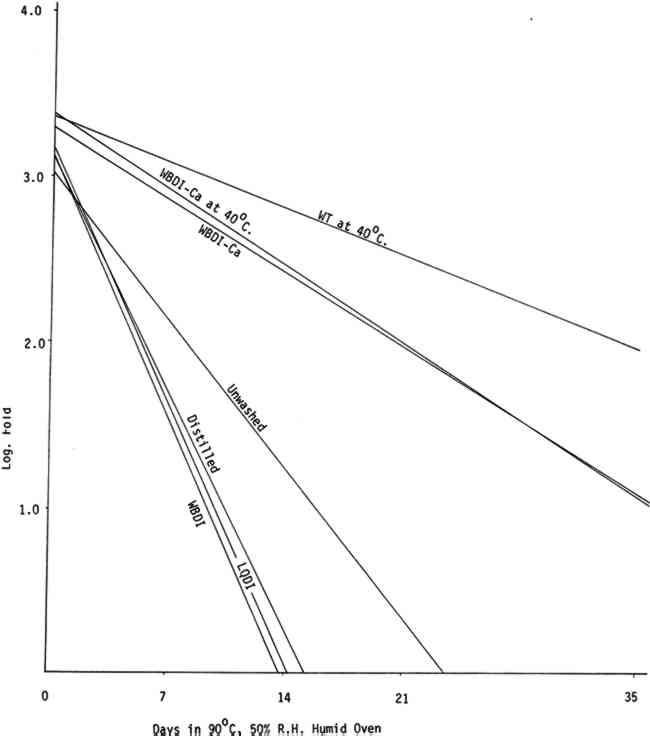
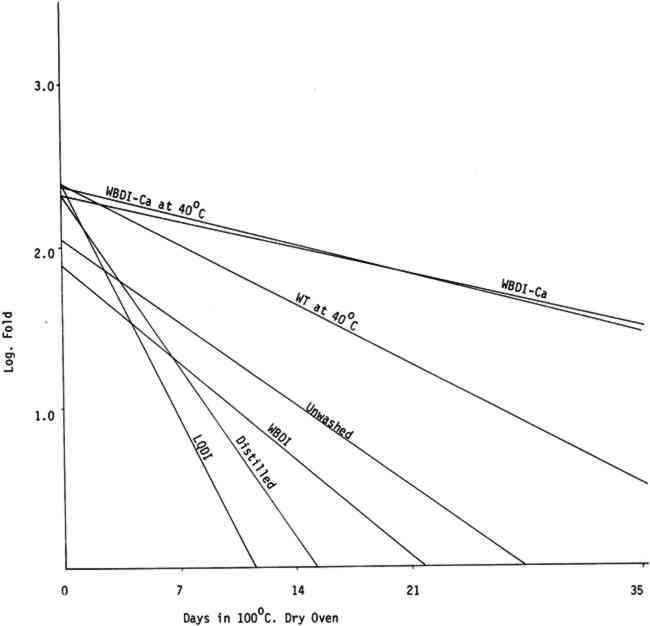
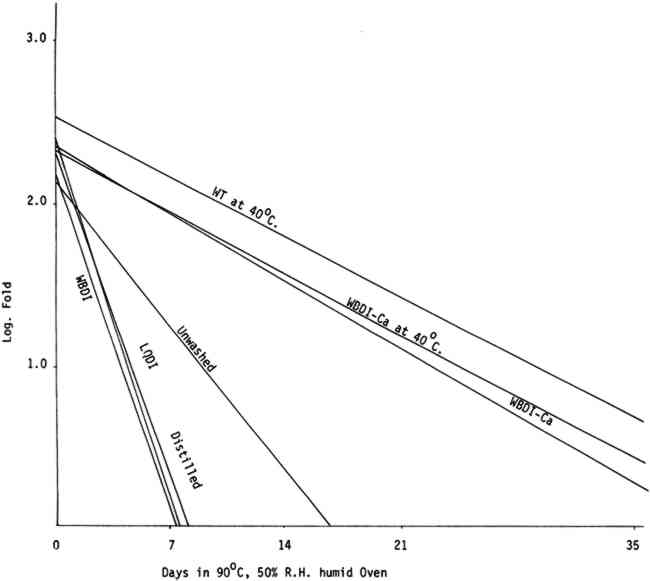
All washing treatments in our experiments increased initial folding endurance. After drying and humid oven aging, the paper washed in the three purest forms of water showed a pronounced tendency to drop in folding endurance when compared with papers washed in Washington tap water and the waters containing calcium and to a lesser extent with unwashed paper controls. That the unwashed controls should retain somewhat higher folding endurance compared with those washed in the purest waters was unexpected (See Tables IIa, IIb, IIIa, and IIIb). Paper samples TABLE IIa FOLDING ENDURANCE OF NEWSPRINT PAPER AFTER VARIOUS WASHING PROCESSES 100�C. DRY OVEN ACCELERATED AGING TABLE IIb FOLDING ENDURANCE OF NEWSPRINT PAPER AFTER VARIOUS WASHING PROCESSES 90�C. 50% R. H. HUMID OVEN ACCELERATED AGING TABLE IIIa FOLDING ENDURANCE OF FOLDUR KRAFT PAPER AFTER VARIOUS WASHING PROCESSES 100�C. DRY OVEN ACCELERATED AGING TABLE IIIb FOLDING ENDURANCE OF FOLDUR KRAFT PAPER AFTER VARIOUS WASHING PROCESSES 90�C. 50% R. H. HUMID OVEN ACCELERATED AGING
The relative life expectancy of paper washed in WBDI-Ca, WBDI-Ca at 40�C, and WT at 40�C varied with the aging method. In dry oven aging, the newsprint and Foldur Kraft samples washed in WBDI-Ca or in WBDI-Ca at 40�C had twice the life expectancy of paper samples washed in WT at 40�C. After humid oven aging, however, paper samples washed in WT at 40�C retained slightly greater folding endurance than the samples washed in WBDI-Ca at 40�C. There was no significant differences for 1, 2, and 3 hour washings. 3.2 BrightnessTABLES IVa, IVb, Va, and Vb show the effect of various washing processes on paper brightness after both dry and humid oven aging. After dry and humid oven aging newsprint washed in the three purest waters lost about the same number of brightness units as the unwashed paper. Newsprint washed in the three calcium containing water consistently retained more of its original brightness after aging than the unwashed sample. TABLE IVa THE EFFECT OF VARIOUS WASHING PROCESSES ON NEWSPRINT PAPER BRIGHTNESS 100�C. DRY OVEN ACCELERATED AGING TABLE IVb THE EFFECT OF VARIOUS WASHING PROCESSES ON NEWSPRINT PAPER BRIGHTNESS 90�C. 50% R. H. HUMID OVEN ACCELERATED AGING TABLE Va THE EFFECT OF VARIOUS WASHING PROCESSES ON FOLDUR KRAFT PAPER BRIGHTNESS 100�C. DRY OVEN ACCELERATED AGING TABLE Vb THE EFFECT OF VARIOUS WASHING PROCESSES ON FOLDUR KRAFT PAPER BRIGHTNESS 90�C. 50% R. H. HUMID OVEN ACCELERATED AGING Foldur Kraft behaved similarly to newsprint on dry aging; but on humid aging all washed Foldur Kraft was improved in brightness retention in comparison with the unwashed sample. 3.3 Chlorine, Iron, Copper, and Calcium Contents of the Wash Waters with Their Effects on Folding Endurance and Accelerated Aging of the Washed PaperANALYSES OF WASHINGTON municipal water are available from the Washington Aqueduct Division. The metal analyses in water9, 13 and the metal content in papers9 were determined by flameless atomic absorption spectroscopy. Analyses of the water used in the several washing systems are given in Table VI. The metal content of the papers after washing is shown in Table VII. In washed papers the iron content varied between 84ppm and 143ppm, which is not considered significant. TABLE VII IRON, COPPER, AND CALCIUM CONTENT FROM VARIOUS WASHING PROCESSES The paper washed in Washington tap water or in WBDI-Ca water contained twice as much calcium as the unwashed papers. The paper treated with the three purest waters had a slightly lower calcium content than the unwashed paper. The WBDI-Ca water picked up 0.010ppm of copper in passing through about 12 feet of copper pipes. The WBDI-Ca water which passed through the heating tank system picked up even more, 0.037ppm of copper. The WT at 40�C and WBDI-Ca at 40�C contained approximately the same amount of copper, although papers washed in WBDI-Ca at 40�C picked up much more during the washing process. Since copper The only wash water to contain chlorine was the Washington tap water at 40�C. In this 0.6ppm chlorine was found, using the ortho tolidine test with the Hellige comparator. This is a rather small amount of chlorine. There does seem to be an indication in Tables IIa, IIIa, and Figure 3 that it has attacked the cellulose slightly, in comparison with WBDI-Ca or WBDI-Ca at 40 A large change in the aging characteristics of the paper came when the paper was washed in the distilled and deionized waters. This is clearly shown in Figures 3, 4, 5, 6, and the treatment index values of Tables IIa, IIb, IIIa, and IIIb. These purified waters acted to remove the protective calcium from cellulose and thus made it more susceptible to the degradative effects of accelerated aging. Added calcium ions to the deionized water increased the concentration of calcium in the treated samples and thus improved their performance on heat aging. WBDI-Ca by this result, should have given excellent results with the paper, and it did for dry oven aging. Results for humid oven aging, however, were less pleasing. Williams et al.3, Czepiel14, and
3.4 pH of the Paper from the Various Wash WatersTHE pH AND TITRATION values obtained from the washed papers are shown in Table VIII. The three highest purity waters gave paper with pH values from 5.0 to 5.4. The pH values of the paper washed in Washington tap water at 40�C were higher than those washed in WBDI-Ca or WBDI-Ca at 40�C. The higher pH values gave the lower titratable acidities in most cases. TABLE VIII pH AND TITRATION FROM VARIOUS WASHING PROCESSES Although the papers washed in pure waters increased in pH, the predicted life generally decreased. When waters containing calcium were used, the pH increased to A continuation of this study will include testing to determine how the choice of water affects the pH of washed papers during and after accelerated aging. 4 CONCLUSIONWATER USED for washing paper artifacts should be free from chlorine and iron and copper compounds, all of which accelerate the deterioration of cellulose. When these potentially harmful substances are removed by distillation or deionization, however, the solvent power of water is increased. Purified water cannot be regarded merely as inert or unreactive. In fact, samples of papers treated in either distilled or deionized In contrast to purified water, when calcium ions were present in the wash water the treated samples showed a marked improvement in accelerated aging tests, even though the levels of copper and iron were also higher. Washington tap water happens to contain the highest concentration of calcium ions of any of the waters used, even more than purified water passed through a calcium carbonate column, and produced the highest levels of calcium in the washed samples. Therefore, the samples treated in ordinary Washington tap water often performed best in the accelerated aging tests, but the variability and unreliability of tap water both in Washington and in other parts of the country prevent us from recommending it as the preferred type of water. These tests were conducted in relatively new acid papers which also happened to contain small amounts of calcium. Since washing in purified water, however, may actually decrease the calcium content of paper, we recommend that water should not only be purified but also have calcium ions added to promote the permanence of the paper. One method for accomplishing this is to allow deionized water to flow through a column of calcium carbonate chips before using it. All piping used for purified water should be pvc or polyethylene, not metal. ACKNOWLEDGEMENTSWE WISH TO THANK Dr. John C. Williams, Research Officer, and Mr. Peter Waters, Restoration Officer, for their support, guidance, and many practical suggestions during the course of this work. We also wish to express our appreciation to our colleagues in the conservation field who generously answered questions and shared with us their experiences with water washing. REFERENCESHey, M.Report to the Council on Library Resources, (Grant CLR-536). Unpublished (1974). Santucci, L.Problems of Conservation in Museums. Paris: ICOM, p. 187–207 (1969). Williams, J. C., Fowler, C. S., Lyon, M. S. and Merrill, T. L.Advances in Chemistry Series. Washington, D.C.: American Chemical Society, No. 164, p. 37–61 (1977). Cellulose Conference, University of California, 1974. Cellulose as a Chemical and Energy Resource. N.Y: Wiley(1975). Clapp, A. F.Curatorial Care of Works of Art on Paper. Oberlin: Intermuseum Conservation Association (1974). Barrow, W. J. “A Two Year Research Program,” Permanence/Durability of the Book. Richmond, Va., 14–23 (1964) Williams, J. C., Kelly, G. B., and Best, R. L.Method of Deacidifying Paper. U.S. Patent 3,898,356 (1975). Mouradgea d'Ohsson, Ignatius. Tableau g�n�ral de l'Empire othoman, divis� en deux parties, dont l'une comprend la legislation mahometane. Paris: Imprimerie De Monsieur (Firmin Didot) Vol. 2 (1788–1824). Tang, L. C.Journal of the American Institute for Conservation. Vol. 17, No. 2, p. 19 (1978). Kelly, G. B.Archives Et Bibliotheques De Belgique. No. 12, p. 91–105 (1974). Barrow, W. J. “A Two Year Research Program,” Permanence/Durability of the Book. Richmond, Va., p. 21–22 (1964). Browning, B. L. and Wink, W. A.TAPPI, Vol. 51, No. 4, 156–163 (1968). Sampson, R. L.American Laboratory. p. 81–84 (1977). Czepiel, T. P.TAPPI, Vol. 43, No. 4, p. 289–299 (1960). Spinner, I. H., Mackinnon, M. H., and Lilley, J. W.Pulp and Paper Magazine of Canada, T144–T118 (1966).
 Section Index Section Index |