Bookkeeper® for Spray Use in Single Item Treatments
by Terry Boone, Lynn Kidder, and Susan RussickIntroduction
The investigation into a new non-aqueous deacidification system for use in single item treatments was based on the premise that deacidification benefits paper based materials and that a conservator's acceptance of these benefits can be compromised by dissatisfaction with currently available non-aqueous methods. The decision to explore the efficacy of the Bookkeeper spray system was supported by encouraging results in the Bookkeeper Mass Deacidification System research1. The goals were to clarify the extent of benefits and risks associated with Bookkeeper and to develop a protocol for selection, testing and treatment of rare and special collection materials.
Several tests were developed while working closely with Dr. Chandru Shahani, Chief, Preservation Research and Testing Division, Ken Harris, Preservation Projects Director, and the Library's Preservation Research and Testing Laboratory staff. These tests were designed to determine the efficacy of the product when applied by spray to single items through measurement of the quantity of alkaline reserve deposited by the spray, the uniformity of the spray application, the speed and extent of the reaction, the amount of penetration or migration possible, and any effect on media.
The Decision to Deacidify
Although much information and knowledge exists regarding the benefits and risks of deacidification, many conservators have not found it easy to interpret and use that information in a consistent manner. Conservators are responsible for the immediate and long term consequences of deacidification treatments, and naturally have reservations about performing treatments which cause unacceptable alterations in the object even though treatment may prolong the life of the object. Physical and chemical alterations are direct consequences of treatment; therefore the intention is to use methods which provide the highest level of benefit while posing the least amount of risk to the object. In the past, potential negative effects of deacidification have manifested themselves as yellowing, textural change or color shift. Understandably, reluctance to deacidify leads some conservators to rely on good housing and storage in lieu of deacidification. These remedies may be appropriate for specific situations; however, they are only partial solutions and do not provide maximum benefit for many objects. Treatment decisions require accountability and need to thoroughly address the real consequences, including the long term costs of partial or sub-optimal treatment solutions. The Bookkeeper spray product is a viable option because testing shows that the chemistry appears to work as theorized with few unwanted side effects.
The treatment referred to as "deacidification" actually describes two different situations: one, the removal of acids, by-products and neutralization of remaining acids, and two, the deposit of an alkaline material which neutralizes the acids and leaves an alkaline reserve. When the need for deacidification is indicated as part of a treatment strategy the conservator must choose between aqueous and non-aqueous methods. In either system, water is necessary for deacidification to occur. The most benefit to the cellulose chain can be gained from aqueous processes because through immersion, water soluble acids and their by-products are actually washed from the paper at the same time that neutralization and deposit of an alkaline reserve occurs. Where aqueous treatment is not possible, neutralization and deposit of an alkaline reserve can still be achieved using a non-aqueous process. Non-aqueous systems all rely on the natural moisture content of the paper and ambient humidity to allow the neutralization reaction to proceed. There are several non-aqueous deacidification systems available. All of these pose concerns for the object, the conservator and the environment. Additionally, some of the chemicals are costly and face government restrictions on usage and disposal. Various formulations have been known to leave an odor or visible surface deposit, cause changes in media solubility or color, cause discoloration of paper, or alter the feel and drape of a sheet. (Bookkeeper does not leave an odor on treated objects, but in some cases a velvety feel is evident when handling treated paper.) Furthermore, the unpredictable effects of application method and equipment also influence the conservator's willingness to deacidify. A particular concern has been the question of "preferential aging", or spots and streaks caused by uneven deposits which age at different rates and are thought to create areas of varying strength and weakness. Aging tests looking for evidence of application methods on preferential effects have not been completed as of this publication.
In 1993 and 1994 the Library contracted with a team of technical experts to evaluate the effects of deacidification with the Bookkeeper mass system. Standard Library of Congress blue test books and a wide variety of bound volumes representing typical Library collection materials were treated by immersion in Bookkeeper. The blue books were then examined, aged and tested for physical endurance. The testing showed that Bookkeeper deposited an adequate alkaline reserve and the treated samples sustained greater fold endurance and tear strength compared to untreated samples. Evaluation of the book components of every test volume (cover, materials, titling, binding, paper, media, etc.) revealed a uniform deposit and no unacceptable evidence of treatment. Since 1995, about 150,000 books at the Library of Congress have been treated with the mass process. The Bookkeeper Spray system has been in use for the last few years on a wide range of collection materials at a growing number of institutions worldwide.
The Bookkeeper non-aqueous deacidification method uses a non-toxic, inert liquid (perfluoroalkane), containing sub-micron sized particles of magnesium oxide with the addition of a surfactant ( a perfluoropolyether derivative) to aid in the dispersion of the particles. When the product is sprayed onto the object, the carrier and surfactant rapidly evaporate and magnesium oxide particles remain lodged in the paper fibers. The acid neutralization process begins, in theory, when ambient moisture or water inherent in the paper reacts with the magnesium oxide to form magnesium hydroxide.
Methodology
Fig, 1. Bookkeeper Spray System
The Bookkeeper Spray System consists of an air compressor, and a two gallon metal container connected to a six foot hose attached to a metal spray gun that produces a flat 70 ¡ fan of spray (fig. 1). The primary area of spray deposit is about six inches wide, bordered by approximately two inches of lighter pattern on either side.
The medium spraying technique used in all the tests was designed to replicate a conservator's standard treatment approach. Working in the fume hood, the spraying parameters were defined as follows: all spraying traveled across the paper in a horizontal pattern at a rate of approximately 2 inches per second, at a distance of 8-10 inches from the object. The technique is to overlap at the edges of the arc in order to create an overall even application. The spraying began and ended off the object. The "light" spray was defined as spraying half the amount of time of the medium application, and the "heavy" spray was two applications of the medium.
Because each of the tests had a separate methodology and results, each will be discussed briefly with conclusions summarized at the end.
Alkaline Reserve Test
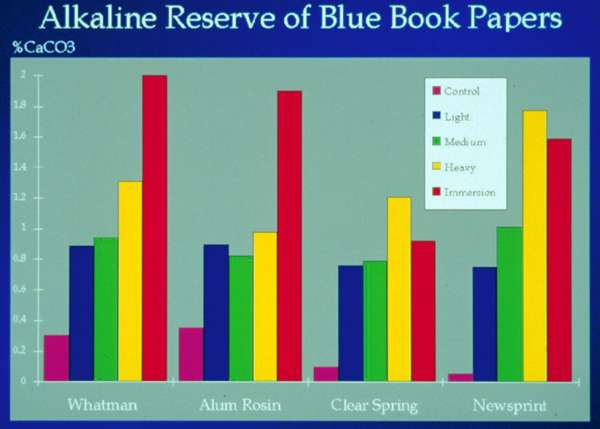
Fig. 2b. Alkaline Reserve of Sprayed Samples of Blue Book Papers vs. Samples Immersed in Mass System
A test was designed to quantify how much magnesium was deposited with spray applications. A comparison with the results from the mass immersion system would allow one to extrapolate longevity expectations for treated objects. Test procedure was to select four papers from the standard blue test books that the Library of Congress had prepared for testing mass deacidification. The papers, Whatman chromatography, Clearspring offset, alum rosin sized, and newsprint, all had a pre-treatment pH below 7. Fiber content respectively included cotton, softwood Kraft, and chemical and mechanical wood pulp. The samples were sprayed on both sides with light, medium and heavy applications. pH was measured and alkaline reserve titrated using TAPPI standard procedure #T553 PM-92 (fig. 2a). Figure 2b shows that the sprayed alkaline reserve deposit was comparable to the mass immersion samples. The vertical axis represents increased percentage of alkaline reserve expressed in terms of CaCO3 and each set of columns represents one of the four papers. A medium spray deposited close to 1% alkaline reserve on the four sample papers.
Uniformity of Deposit Test
Two tests were designed to visually evaluate the degree of uniformity of the spray deposit. Test "A" was simply to spray samples of black paper with light and heavy applications and compare them with a sample which was individually immersed. The lightly sprayed sample showed no visual evidence of treatment. The sample which was heavily sprayed remained uniform overall but no longer appeared as densely black as the unsprayed sample. Particles were not distinctly visible on the sprayed samples, because when properly dispersed they are too small to be seen under low magnification2.
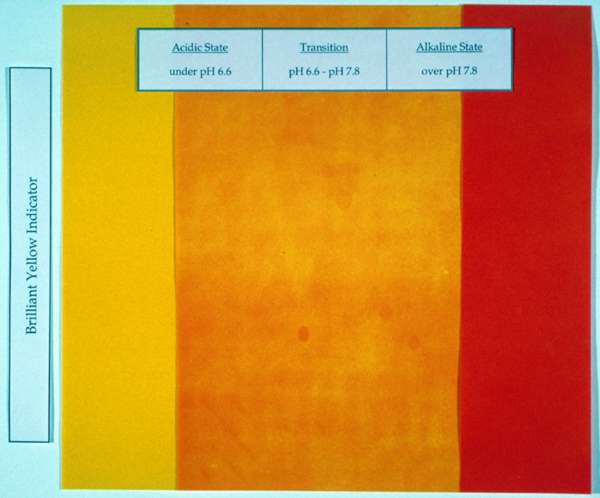
Test "B" was designed to reveal the pattern of magnesium deposit created by the spray nozzle. It was assumed that paper impregnated with a pH indicator solution would instantly record the spray pattern during application . Indicator papers were created by immersing standard Whatman chromatography paper in brilliant yellow indicator solution. In the acidic phase (below 6.6) this indicator is bright yellow and appears red-orange when alkaline (above pH 7.8). The indicator paper samples were sprayed with Bookkeeper solution on both sides with a medium application.
Fig. 3. Color Change in Treated and Humidified Indicator Paper
After spraying the treated indicator samples remained yellow and did not undergo the anticipated color shift indicating alkalinity. The samples were then placed between blotters and stored at room temperature for a few days to allow inherent moisture in the paper and ambient humidity to cause the change, but still no change was observed. The decision was made to accelerate the process by humidifying the samples to reveal the spray pattern. Within 5-15 minutes in a warm water passive humidity chamber, the color changed gradually, ultimately reaching the red-orange color in about an hour (fig. 3). A relatively even spray pattern was evident during the early stages of humidification. However, as humidification progressed, the pattern disappeared and the sheet became a solid color. Occasional areas of variable quantities of deposit (such as those caused by drips and sputters) were not visible on the fully humidified samples.
Speed and Extent of Neutralization Reaction Test
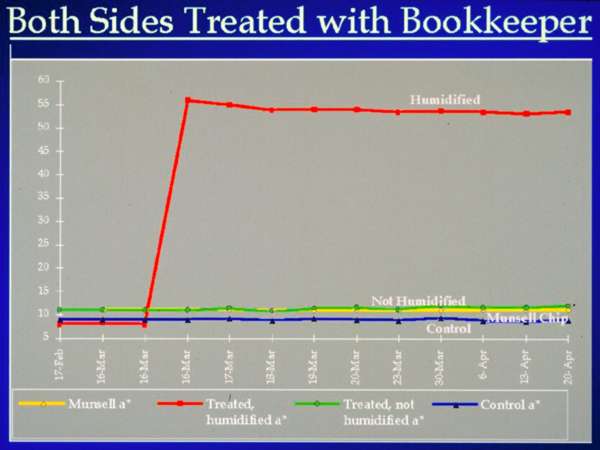
Fig. 4. Comparison of Colorimeter Readings of Humidified and Unhumidified Treated Samples
The 1994 report states that "the chemistries leading to acid neutralization are poorly understood and occur over some indefinite time period following the treatment"3. The color change in the brilliant yellow indicator paper to orange is a pH sensitivity reaction The lack of a change suggests that if neutralization occurs the pH of the paper is less than or equal to 6.6. Significant moisture (passive humidification in this instance) had to be introduced in order to cause the pH change in these alkaline sensitive test materials. A test was designed to measure the speed and extent of the neutralizing action because the expected color change did not appear to be occurring in the indicator papers without exposure to additional moisture. The Minolta Chroma Meter CR-221 was used to record color change over time because changes in the unhumidified samples were so subtle. Six groups of indicator papers were tested, some sprayed with the product on one side and some on both sides. Half of the samples were humidified and half were not. Dramatic color change occurred in treated samples which had been humidified, while there was no visual evidence of change in those that had not been humidified. Figure 4 compares results of two different samples, one humidified and the other not, where the colorimeter readings were taken before treatment and over a five week period after humidification. The measured color change over time was insignificant in the unhumidified samples. Humidification definitely accelerated the rate and the extent of the color change in the samples. There was a sharp initial increase in redness at the time of humidification which then leveled off and remained fairly constant.
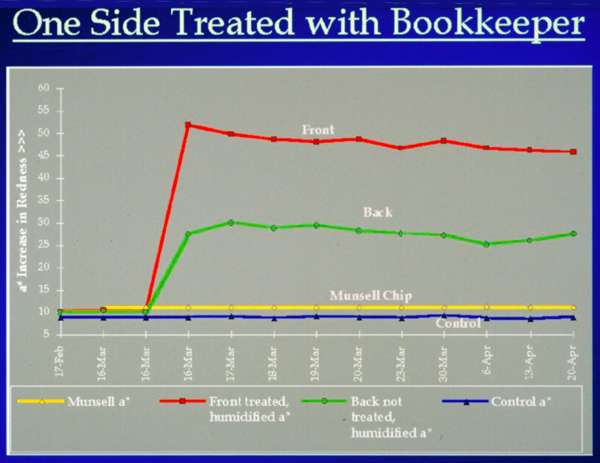
Fig. 5. Comparison of Front and Back of Humidified Single Sample Treated on Front Only
Figure 5 compares the colorimeter readings from the front and back of a single sheet which was treated only on the front and then humidified. While a dramatic increase in redness occurred on the treated side, there was also a significant increase in redness on the untreated side. Penetration from the treated side of the sample through to the untreated side was unexpected since the magnesium is felt to be too large to move into the paper itself. Observation of what might indicate penetration led to the development of the next test.
Degree of Penetration Test
| Layer No. | 0 Hour | 1 Hour | 24 Hours |
|---|---|---|---|
| 1 | 10.1 | 10.3 | 9.4 |
| 2 | 6.8 | 6.5 | 6.3 |
| 3 | 5.4 | 5.6 | 5.6 |
| 4 | 5.3 | 5.6 | 5.5 |
| 5 | 5.4 | 5.5 | 5.5 |
| 6 | 5.3 | 5.4 | 5.4 |
Fig. 6a. pH of Newsprint Block Samples
| Humidification Time | |||
| Layer No. | 0 Hour | 1 Hour | 24 Hours |
|---|---|---|---|
| 1 | 1.693 | 1.926 | 0.79 |
| 2 | 0.083 | 0/137 | 0 |
Fig. 6b. Alkaline Reserve of Newsprint Block Samples
| Humidification Time | |||
| Layer No. | 0 Hour | 1 Hour | 24 Hours |
| 1 | 10.64 | 10.97 | 11.01 |
| 2 | 10.32 | 10.96 | 10.87 |
| 3 | 4.88 | 5.4 | 10.74 |
| 4 | 4.33 | 4.69 | 9.75 |
| 5 | 4.31 | 4.65 | 4.7 |
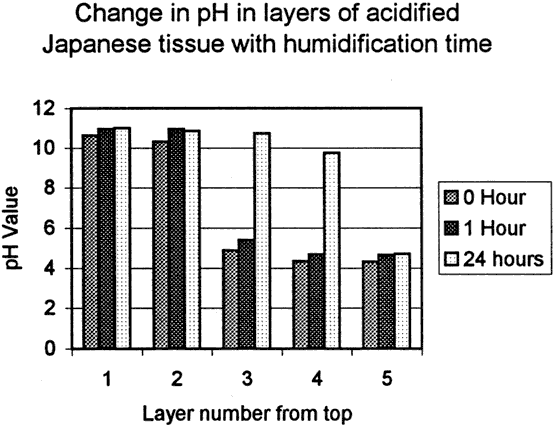
Fig. 7a. pH of Japanese Paper Block Samples
| Humidification Time | |||
| Layer No. | 0 Hour | 1 Hour | 24 Hours |
| 1 | 4.722 | 5.138 | 5.132 |
| 2 | 2.005 | 2.076 | 2.775 |
| 3 | 0 | 0 | 0.697 |
| 4 | 0 | 0 | 0.077 |
Fig. 7b. Alkaline Reserve of Japanese Paper Block Samples
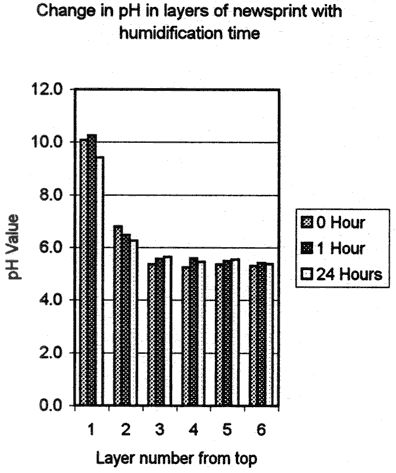
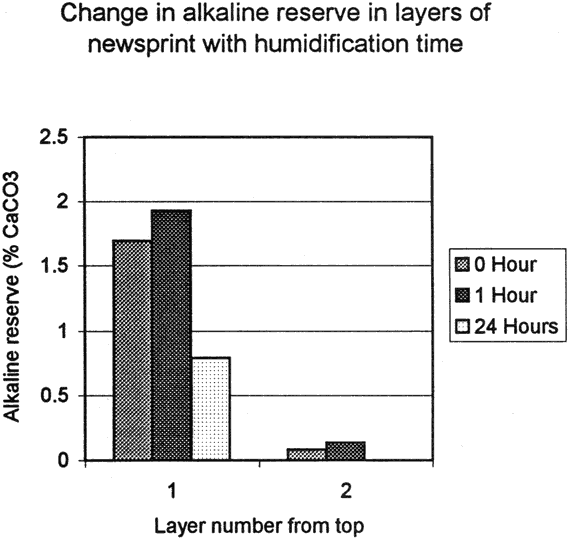
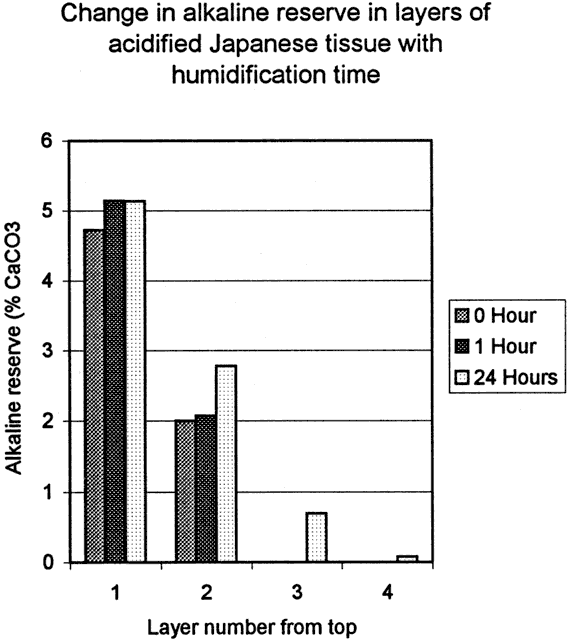
The fourth test was designed to determine the degree of penetration of Bookkeeper into the paper. Two sets of sample blocks were made, one of acidic newsprint (ca 1970's) and one of acidified Japanese paper (ca. 1990's). The newsprint blocks were made by layering six sheets wet out with water then pressed together. The Japanese blocks were made up of nine sheets, also wet out and pressed together. When dried, the blocks were sprayed with the Bookkeeper product on one side. The blocks were then edge trimmed and cut into twelve sample blocks. The samples were humidified for one hour, or 24 hour periods, or not at all. After humidification, the sample blocks were dried under pressure in order to keep the layers in contact with each other, then they were disassembled, and each layer was titrated separately for alkaline reserve and pH (fig. 6, 6a and fig. 7, 7a).
Both sample groups showed significant alkaline reserve in the top layer, some alkaline reserve in the second layer and a corresponding rise in the respective pH. Initial test results of the Japanese paper blocks show a significant alkaline reserve on the second layer as well as measurable amounts on layers three and four. Due to time constraints the number of our samples was limited and therefore additional data needs to be collected before further conclusions are drawn regarding Japanese papers.
The newsprint samples showed significant alkaline reserve in the top layer, two of the samples showed a measurable alkaline reserve in the second layer and all samples had an elevated pH in the top and second layers. These numbers indicate penetration of the alkaline material through the first layer of newsprint.
There was no significant difference between the alkaline reserves and pH Values between the one hour humidified and unhumidified samples, which indicates penetration occurred at the time of application. However the 24 hour humidification did cause change, resulting in lower alkaline reserves and pH
Values. The lower alkaline reserve results are significant because they indicate that the alkaline reserve has been partially used, yet a measurable amount remains in the sample. The pH level in the top layer has correspondingly been slightly reduced, but remains comparable to that of the samples which were not humidified and those which received an hour of humidification.
Test for Effect on Media
Figure 8. Media and Materials Treated and Tested in the Bookkeeper Mass System
- Covering case/materials
- buckram
- pyroxylin bookcloth
- vinyl/plasticized cloth and paper
- leather
- cardweight paper
- printed paper
- coated paper
- color foil stamping
- gold foil stamping
- gilded edges
- pressure sensitive labels
- bar codes
- plastic adhesives
- pyroxylin bookcloth
- Textblocks
- newsprint
- alum rosin sized paper
- alkaline pulp paper
- bond weight paper
- lightly to heavily calendered paper
- coated paper stock
- glassine
- alum rosin sized paper
- Structures
- machine sewn
- oversewn
- post binding
- staples
- plastic spiral binding
- perfect binding
- oversewn
- Media
- various black and colored printing inks dating from the1800's
and 1900's.
- ball point pen ink
- pencil
- felt tip pen ink
- highlighter marker ink
- ball point pen ink
- Extraneous Materials
- bookplates
- plastic coated paper clips
- rubber bands
- plastic coated paper clips
A wide range of media has already been tested in the mass project (fig. 8). Although the Bookkeeper product has not been found to cause bleeding or sinking of any media, pH related color shifts are of course possible. The one example of a pH color shift that was observed was in the mass immersion process where a blue highlighter marker turned green (without humidification) as a result of treatment with Bookkeeper. In these final tests media was selected which was specific to rare materials, including some known to be alkaline sensitive (fig. 9). All were commercially produced watercolor or gouache except for the gamboge and copper acetate/verdigris, which were made following historic recipes. Bookkeeper was applied to samples as follows: no application, light on one side, light on both sides, heavy on one side, heavy on both sides or immersed. When treated on only one side the Bookkeeper was sprayed directly onto the media side of the sample. Again, sample groups were humidified either for one hour or 24 hours or not at all.
Figure 9. Media Tested With Bookkeeper Spray
Alizarin crimson
Alizarin rose madder
Cadmium yellow medium
Chrome yellow
Gold metallic drawing ink with gum arabic
Hooker s green deep
Indian yellow
Orange lake deep
Prussian blue
Raw sienna
Ultramarine deep
Van Dyke brown
Verdigris
Vermilion
Results showed that some of the darker colors which had received a heavier application appeared to be very slightly grayer or hazier than the same colors which got light and medium applications. This effect is not specifically a change in the media, but the result of white deposit on a dark or solid colored field. Most of the samples did not undergo a noticeable change, with the exception of gamboge. Samples of gamboge which were treated but not humidified remained yellow. After humidification, gamboge displayed a textbook example of a pH color shift. It was interesting to note that the color shift to orange was complete on the sample which had Bookkeeper lightly applied on both sides and on those with the heavier applications, yet the samples which were lightly treated on one side and humidified were still very true to the original yellow color.
Additional media and papers which have been treated are listed in Appendix 1.
Conclusions and Recommendations for Use
To summarize, the intention was to determine the efficacy of the product when applied by spray to single items, to measure the quantity of alkaline reserve deposited by the spray, the uniformity of the spray application, the speed and extent of the reaction, the amount of penetration or migration possible and any effect on media. In general it appeared that the equipment applied the product effectively, uniformly and deposited an adequate alkaline reserve, and produced minimal effect on a wide range of media. It also appeared from this work that the product penetrated into the test papers, and that humidity played a role in the process of using the alkaline reserve.
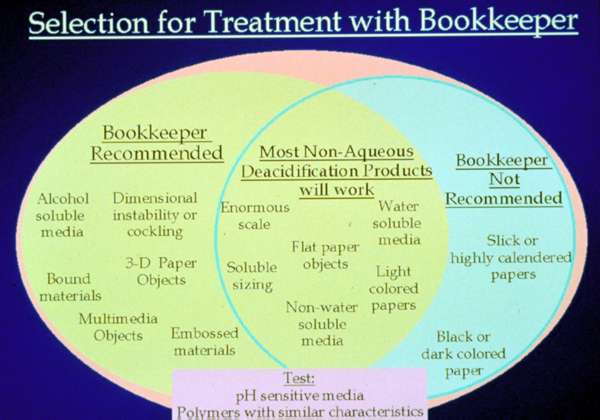
Fig. 10. Selection for Treatment with Bookkeeper
A conservator would consider the needs of the object when interpreting any test results and incorporating the information into actual treatment (fig. 10). Tests showed that the system will deposit an adequate alkaline reserve when applied to most papers using a medium spray, but of course, the spraying technique should be customized to the paper and media. For example, a more open paper like Japanese, whether by fiber formation, or breakdown of size or age, will be penetrated more easily than a paper of greater weight, harder surface or fresher size.
In order to obtain the most significant alkaline reserve and penetration it is recommended that most papers be sprayed on both sides. Penetration occurs as a result of wetness at the time of spraying and the spray should be applied with this in mind. Post-treatment humidification is a step to consider because it accelerates the acid neutralization process, but tests showed that measurable deacidification will occur to some extent even without humidification.
The behavioral evidence of the chemistry of Bookkeeper on pH sensitive media allows the conservator greater latitude in treatment decisions.
Bookkeeper poses minimal risk to most of the media that was tested. However, it is clear that in order to witness potential pH color shifts both Bookkeeper and moisture must be applied. The risk of color shift is greatly reduced if less of the product is used, or if it is applied to the verso. Bookkeeper has been applied successfully to most papers, however the product is somewhat evident on dark colors and highly calendered papers. Layering on lightly would be an option when the color or surface quality of the paper or media will cause a deposit to be more visible. Documentation of objects treated with Bookkeeper is important since future treatment or water emergencies may result in color shift.
Although Bookkeeper is non-toxic, fine particulates are involved which may result in lung or eye irritations, and the use of gloves and the fume hood are recommended for this reason. Because of the rapid drying time, low reactivity, and non-toxic nature of the carrier, items may be removed from the fume hood immediately after spraying. Batched treatments or bound materials may be stacked or closed without fear of distortion or offsetting.
More extensive testing of media treated with Bookkeeper will allow expanded protocol for selection. Research needs to be undertaken on potential reactions with graphite, metallic printing inks, and other media conservators encounter in their work.
While Bookkeeper will not solve all non-aqueous deacidification problems, the Bookkeeper spray system provides a safe, effective and easily used method which causes virtually no evident harm to the environment, the user and the object.
Acknowledgment
Special thanks to our colleagues at the Library of Congress, especially
Dr. Chandru Shahani, Ken Harris, Marta-Lucia Sierra, Andrew Robb, and Tom Albro. Also Karen Pavelka from the University of Texas for media samples.
Notes
1. Bennett, W., Buchanan, S., Domach, M., Melnick, S., Tancin, C., Whitmore, P. Evaluation of the Bookkeeper Mass Deacidification Process, Technical Evaluation Team Report for the Preservation Directorate, Library of Congress. Preservation Directorate, Library of Congress, Washington, D.C., 1994.
2. Because the alkaline deposit resulting from mass immersion appears uniform on white paper before and after accelerated aging, by visual observation and with magnification it seemed possible that immersion of a single sheet would provide a similarly uniform deposit. When immersed and air dried, the product puddled on the paper, resulting in a blotchy appearance and a visible white deposit. When viewed under .8-64X magnification, clumps of magnesium particles were evident along the tidal edge of the puddling on the immersed sample.
3. However, in Appendix E, PTI presents statistical evidence of the rate of deacidification. Bennett, W., Buchanan, S., Domach, M., Melnick, S., Tancin, C., Whitmore, P. Evaluation of the Bookkeeper Mass Deacidification Process, Technical Evaluation Team Report for the Preservation Directorate, Library of Congress. Preservation Directorate, Library of Congress, Washington, D.C., 1994.
References
Derek Page et al, "Method for the Deacidification of Papers and Books" Paprican, Canada. In Abbey Newsletter, Volume 19, Number 6-7, Dec. 1995
Couch, Randall, compiler. Alkalization and neutralization. In Paper Conservation Catalog, Chapter 20. 2nd ed. Washington, D.C.: American Institute for Conservation of Historic and Artistic Works, Book and Paper Group, 1985.
IPST. Physical Properties of Library Books, Untreated Control Books. Report #4 to Contracts and Logistics RFP90-32. Atlanta, Georgia, June 1991.
Lehner, S., revised and compared with the 7th German Edition by Mitchell, C. Ainsworth. Ink Manufacture.. Scott, Greenwood and Son, London, 1926.
Mayer, Ralph. Artist's Handbook. Viking Press, New York, 1957.
Mitchell, C. Ainsworth and Hepworth, T.C., Inks; Their Composition and Manufacture,. London. Charles Griffin and Co. LTD. 1916.
Roy, Ashok, Editor. Artists' Pigments, A Handbook of Their History and Characteristics. Vol. I. National Gallery of Art, Washington, D.C. 1993.
Appendix 1: Special Collection Materials Treated With Bookkeeper Spray
In 1997 and 1998 over two thousand leaves were treated with Bookkeeper spray. These manuscript and printed collection materials date from 1874 to the 1990's. Approximately 100 various media and papers are represented. No evidence of alteration to any of the media or paper types was observed during any of the spot testing or treatment.
1874-1883 written and printed correspondence
(personal and business).
Over 550 items spot tested and treated with no evident alteration of
media or paper.
- Media
- copy press purple ink
- fountain pen ink -dark blue, light
- white and dark brown, purple, red, and turquoise blue
- intaglio printed gold and silver ink
- iron gall ink
- lined paper ink—blue, red
- manuscript ink—black, brown
- newsprint ink
- pencil
- printing ink—black, brown
- printed postal stamp ink—yellow, green
- shellac/wax seal
- stamp pad—purple
- telegraph printed type—blue
- typewriter ribbon—blue, black, purple
- fountain pen ink -dark blue, light
- Paper
- bond paper—lined, invoice letterhead
- calendered stationers paper—buff,
- graph paper—lines created by paper mould
- onion skin
- stationers paper—embossed stamp
- text weight paper—blue, white, blue
- lined legal, cream colored calendered
- Whatman 1870 white
- transparentized paper
- calendered stationers paper—buff,
1893-1894 written and printed correspondence
(personal and business).
Over 250 items spot tested and treated with no evident alteration of
media or paper.
- Media
- fountain pen—black, blue, brown
- pencil
- printed ink—blue
- stamp pad ink
- pencil
- Paper
- bond paper—calendered, heavy weight
1925-1930 written and printed correspondence
(government and business).
Over 100 items spot tested and treated with no evident alteration of
media or paper.
- Media
- carbon paper—black, manuscript and typed
- fountain pen—black
- printers ink—black
- fountain pen—black
- Paper
- text weight—blue
- onion skin—bond weight
- onion skin—bond weight
1949 written and printed correspondence
(government and business).
Over 100 items spot tested and treated with no evident alteration of
media or paper.
- Media
- carbon paper—black, typed
- fountain pen ink—black
- typewriter ribbon ink—black,
- turquoise
- fountain pen ink—black
- Paper
- bond weight—laid with watermark"Royal Seal Bond, USA",
typing paper
- bond weight—onion skin, typing paper
- card weight stock—foam green
- bond weight—onion skin, typing paper
1930-1995 written and printed correspondence
(personal and literary publication business).
Collection of 6000 items, over 120 items spot tested with no evident
alteration of media or paper. approximately 100 items treated,
treatment will continue when staff and funding allow.
- Media
- Ball-point pen—blue, other
- Carbon paper—black, blue
- Computer printer—dot matrix
- Crayon—red
- Felt-tip pen—black, green, other, teal/turquoise
- Fountain pen—black, blue, brown
- Pencil—blue, graphite, red
- Photocopy
- Printing ink—black, blue
- Typewriter ribbon—black, red
- Carbon paper—black, blue
- Paper
- Bond—blue, green, high lignin/aged to brown, white,
yellow: Category of cotton and/or chemical woodpulp papers
commonly used for writing, printing and typewriting, characterized
by medium weight, uniform finish, smooth surface, and the absence of
ruling. Bond includes white, yellow, blue and green papers in
various sizes (letter, legal, etc.) as well as letterhead
stationery. N.B. This collection contains a bulk of letter size
high-lignin bond paper (original color undetermined) which has
become brown and brittle with age, as well as other which have
retained their color, strength and flexibility.
Computer Printout Paper: Category of light to medium weight chemical wood and/or reclaimed pulp papers used in printers associated with computers. These papers are characterized by fan-folding for continuous-feed printing and perforations to allow for separation of sheets after printing.
Napkin Paper: A gauzy, fairly transparent tissue made of a variety of pulps and used in the manufacture of paper napkins.
Onionskin Paper: Category of lightweight, semi-translucent cotton and/or chemical woodpulp papers used for writing, typing, and in particular the making of carbon copies. Onionskin is characterized by the absence of ruling and smooth, glazed, plated or supecalendered finishes. The category includes white, yellow, and bluepapers in various sizes.
Rules Paper: Category of utilitarian chemical woodpulp papers used for writing or typing and characterized by light to medium weight, smooth finish, and horizontal lines (with or without vertical margins) ruled in blue and/or red ink. Includes white and yellow papers in letter, legal, and other formats, hole-punched, spiral- or tablet- bound and loose-leaf. Due to the effects of aging the original color of some papers in this category may be impossible to determine.
Thermo Fax (Wet process photocopy): A stark white, slippery-surfaced, coated paper manufactured for use in wet-process photocopying.
Senior Rare Book Conservator
Library of Congress
Lynn Kidder
Senior Rare Book Conservator
Library of Congress
Susan Russick
Book and Paper Conservator
The Newberry Library
Publication History
Received: Fall 1998
Paper delivered at the Book and Paper specialty group session, AIC 26th Annual Meeting, June 1-7, 1998, Arlington, Virginia.
Papers for the specialty group session are selected by committee, based on abstracts and there has been no further peer review. Papers are received by the compiler in the Fall following the meeting and the author is welcome to make revisions, minor or major.