The Use of Calcium Bicarbonate and Magnesium Bicarbonate Solutions in Small Conservation Workshops: Survey Results
by Amy E. Gerbracht and Irene BrückleAbstract
The treatment of acidic paper with aqueous alkaline solutions has been the subject of intensive investigations in the past. the present study focused on some questions regarding the uses of various washing solutions by paper conservators. A mail survey was sent out to seventy-six paper conservators to ascertain what types of aqueous treatments have been or currently are being carried out in conservation labs. the survey respondents were asked what particular washing solutions have been abandoned over the years and why their use was discontinued. the responses to this questionnaire revealed that the use of both calcium bicarbonate and magnesium bicarbonate solutions has diminished and provided answers as to why this is the case.
Introduction
Calcium and magnesium bicarbonate solutions have long been utilized in paper conservation for washing treatments.1 These solutions are useful as they not only neutralize existing acids in paper, as does, for example, ammonium hydroxide, but because they also impart an alkaline reserve of calcium or magnesium carbonate to paper, helping to protect it from future deterioration. Informal discussions of the authors with paper conservators suggested that in more recent years, however, the use of these solutions has declined. To determine if this is indeed the case at present, a survey on the uses of these washing solutions was sent out to seventy-six paper conservators in the United States and abroad. the questionnaire was mailed to conservators treating works of art as well as those treating archival collections. Recipients included those who work in private practices, museums, and regional centers.
Survey
Those surveyed were asked about their past experiences with each of the following four alkaline washing solutions: ammonium hydroxide, calcium hydroxide, calcium bicarbonate, and magnesium bicarbonate. Forty paper conservators (53%) responded to the questionnaire and thirty-eight are performing or have performed aqueous treatments on works of art and/or archival materials. of these 38 respondents, 35 treat works of art (13 only treat works of art and 22 also treat archival objects) and 25 carry out treatment on archival objects (3 treat only archival objects and 22 also treat works of art):
Number of respondents who treat:
| only works of art | 13 | (34%) |
| only archival objects | 3 | (8%) |
| both works of art and archival objects | 22 | (58%) |
Four respondents indicated that they work additionally with other materials such as photographs, papyrus, and amate.2
Conservators were asked which of the four solutions they currently use for the washing/deacidification of paper materials. Their answers are summarized in Figure 1.
Overall, calcium hydroxide and ammonium hydroxide are used much more frequently than the bicarbonate solutions. the survey results also indicated that both works of art and archival objects are treated with similar frequency with calcium hydroxide or ammonium hydroxide solutions. Archival objects undergo magnesium bicarbonate treatments slightly more frequently than works of art, whereas the use of calcium bicarbonate is relatively rare for either type of material (see percentages in Fig. 1).
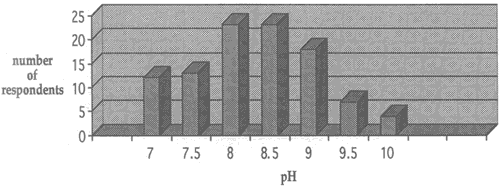
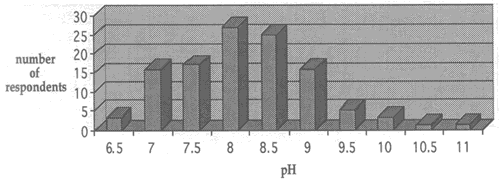
Fig. 2. Range of solution concentations for washing with ammonium hydroxide
Fig. 3. Range of solution concentations for washing with calcium hydroxide
Regarding solution concentration, the paper conservators who responded to the survey reported using a wide range of pH values when carrying out treatments with calcium hydroxide and ammonium hydroxide. Concentrations for both solutions were determined by bath pH (the concentration of the reagents were adjusted until the desired pH was reached). the range of solution concentration for calcium hydroxide was between pH 6.5 and pH 11.0 and for ammonium hydroxide between pH 7.0 and pH 10.0. Figures 2 and 3 show the choices of solution concentrations indicated by respondents.
Almost all of the respondents who use the higher pH solutions stated that they did so only for localized treatment of stains and not for immersion washing. There seems to be a general agreement among respondents that the washing treatments with ammonium hydroxide and calcium hydroxide are best carried out in the range between pH 7 and pH 9. A pH lower than 7 does not reduce acidity in deteriorated paper effectively, and the survey confirms that pH levels above 9 are usually avoided because of potential negative effects on media and paper constituents.
There were a number of reasons given why calcium hydroxide and ammonium hydroxide are currently chosen more often than bicarbonate solutions. Fifteen respondents stated that they decide to use the hydroxide solutions due to ease of preparation. Concerning ammonium hydroxide, six conservators said that they use this solution because it leaves no residue in the paper after treatment due to its volatility. of those who currently use calcium hydroxide, many stated that they feel there is less chance of a precipitate forming on the paper surface after treatment when compared with bicarbonate solutions.
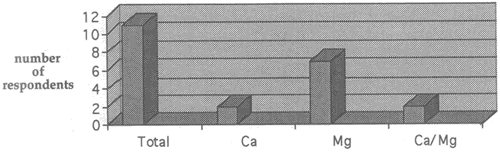
Fig. 4. Number of respondents who currently use calcium bicarbonate, magnesium bicarbonate, or both solutions
Eleven of the thirty-eight respondents currently use either or both bicarbonate solutions. Figure 4 shows which of the two (or both) solutions these paper conservators are using.
It was particularly interesting to note that all but one of the conservators determine the concentration of bicarbonate solutions by measuring the pH of the solution and adjusting it to levels between pH 6 and pH 9, instead of determining ppm calcium or magnesium. the relationship between the pH and the concentration of bicarbonate solution is, however, only a very tentative one, as has been indicated by several authors. Brederick et al. noted the pH level decreases with increasing solution concentrations as carbon dioxide is added (p. 173, Table 3).3 Wilson et al. determined the pH levels of magnesium bicarbonate solutions from which carbon dioxide has been removed: a 1M solution that had an initial pH of 6.98 showed a pH increase to 8.52 after the removal of carbon dioxide (pp. 90-91, Table I).4
Conservators presented a number of reasons why they currently utilize magnesium and/or calcium bicarbonate for aqueous treatments. One respondent observed there is "some feeling that paper tone is better with [magnesium bicarbonate] than calcium hydroxide (which seems to cause more undesirable whitening)." Some conservators pointed out that bicarbonate solutions leave an alkaline reserve in the paper, give longer life to the paper, and provide consistent results time after time.
A few respondents who currently utilize bicarbonate solutions mentioned that they do encounter problems with the solutions. Two conservators reported that they occasionally see a precipitate on papers after treatment with magnesium and/or calcium bicarbonate. This common occurrence, which has been termed "gritting," is owing to excess metal carbonate being left on the paper surface.5 Additionally, one respondent occasionally experiences "some movement of paper discoloration during the drying process." This phenomenon is probably due to the fact that as the solution ages, carbon dioxide contained in it is lost to the air and the pH of the solution increases as a result. the residual solution still contained in the paper during drying therefore increases in pH. Also, as the water evaporates, the concentration of magnesium compounds increases, which may affect an increase of the pH level in the paper. At such an elevated pH, it is possible that weakly-acidic, colored substances in the paper might become more soluble than they were previously under the lower pH conditions prevailing in the bicarbonate bath solution. This may result in the migration of discoloration in the drying paper.
One conservator chooses to use magnesium bicarbonate for washing wood pulp paper to improve its long-term stability. the same conservator is also concerned about the potential darkening effect this type of treatment may have on the ligneous paper constituents, and points out that therefore, solutions of very low (unspecified) concentration are used. However, this conservator is not certain whether at these low concentrations, darkening of the ligneous paper is truly avoided.
Overall, ten conservators responded that when treating wood-pulp papers, they tend to use lower-pH solutions (one specified using no higher than pH 7 for these papers). Six of the ten respondents noted that they use the following solutions when treating wood-pulp papers: calcium hydroxide, ammonium hydroxide, and magnesium bicarbonate. One conservator does not use calcium hydroxide on wood-pulp papers because of its "graying effects." In addition, five respondents said that they use lower-pH solutions when alkali-sensitive media are present (watercolor, printing inks, writing inks). Ammonium hydroxide was mentioned by three conservators as their preferred solution. Another chooses not to use ammonium hydroxide when sensitive media is present as it is "more aggressive in dissolving inks." One respondent keeps all of their washing solutions at or below pH 7 when treating watercolor and other alkali-sensitive colors.
Conservators were also asked which of the four solutions they have used in the past for the washing/deacidification of paper materials but use no longer. Sixteen respondents stated that they currently use the same solutions that they have used in the past. Twenty-two of the thirty-eight respondents used different solutions in the past than they do currently. of these respondents, 19 treated works of art (7 only treated works of art and 12 also treated archival objects) and 15 carried out the treatments on archival objects (3 treated only archival objects and 12 also treated works of art):
Number of respondents who used the solutions to treat:
|
only works of art |
7 |
(~32%) |
|
only archival objects |
3 |
(~14%) |
|
both works of art and archival objects |
12 |
(~58%) |
The responses of the twenty-two conservators who currently use different solutions than they have used in the past is summarized in Figure 5.
As evident in the responses listed in Figure 5, very few conservators have chosen to discontinue their use of hydroxide solutions, showing that most are pleased with the after- treatment results when calcium and/or ammonium hydroxide are used for washing. This does not appear to be the case with calcium bicarbonate and magnesium bicarbonate solutions. of the twenty-two respondents who reported changes in their choice of washing solutions over time, eighteen have used bicarbonate solutions in the past and have since discontinued their use.6 Three of the twenty-two have not stopped using bicarbonates entirely but have limited their use to one of the two bicarbonate solutions, whereas in the past they employed both solutions.
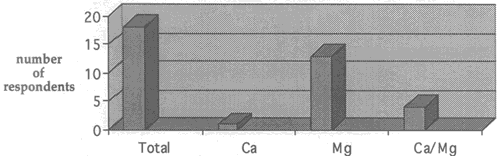
Fig. 6. Number of respondents who have used calcium bicarbonate, magnesium bicarbonate, or both solutions and no longer use them.
As stated, eighteen of the twenty-two conservators who have changed their solution choices over time have chosen to discontinue their use of bicarbonates. Figure 6 illustrates which of the two (or both) bicarbonate solutions were used most often by these conservators in the past and are no longer used by them currently. the chart takes into account both works of art and archival materials.
Overwhelmingly, the response as to why bicarbonate solutions are currently not used for washing as often as are those of dilute ammonium hydroxide and calcium hydroxide is because they are "inconvenient." Bicarbonate solutions were described by respondents as "fussy," "complicated," "troublesome," "difficult," "time-consuming," "expensive," and "awkward." In addition to these problems, many responded that they had experienced undesirable after-treatment results when using bicarbonate solutions. Nine of the eighteen conservators who have discontinued their use of calcium and/or magnesium bicarbonate stated that the solutions left a deposit on the paper surface after treatment that caused a change in the texture and visual appearance overall (gritting). Another respondent felt that there has not been enough research carried out concerning the distribution of the carbonates after drying, and that if the distribution is uneven, discoloration upon aging may occur irregularly as well.
One conservator replied that magnesium bicarbonate solutions caused "unacceptable changes to inks and pigments" and also that they "almost always altered the texture and feel of the paper, leaving it stiff and rough." In addition, three other respondents mentioned the negative effect bicarbonates can have on media. One respondent discontinued using bicarbonate solutions when they started to exclusively treat fine art and feels that it "can be very useful for some archives conservation applications." Another issue which raised concern is the increase in the surface pH of treated papers. Four respondents discontinued their use of bicarbonate solutions due in part to the fact that they found very high surface pH measurements in their treated papers (no pH values were given). Finally, conservators also worried about the potential color alteration of papers treated with bicarbonates. One respondent reported a "definite grayish-blandness to the paper after treatment." Overall, respondents reported that they have experienced papers turning "pink," "orange," "yellow," "brown," "tan," "gray," or generally "cooler" in tonality.
Conclusion
Overall, twenty-nine (76%) of the thirty-eight conservators who replied to the survey have used in the past or currently use bicarbonate solutions. the majority of conservators who have used and/or still are using bicarbonate solutions have chosen to use magnesium over calcium bicarbonate, mostly because it is easier to dissolve magnesium hydroxide or carbonate than it is to dissolve calcium hydroxide or carbonate. (This may be due to the fact that magnesium carbonate is more soluble in water than calcium carbonate.) As a whole, paper conservators who reported using or having used these solutions tended to use them equally on works of art as well as archival materials.
Regarding the unsatisfactory results reported by conservators using bicarbonate solutions (occurrence of gritting, the movement of discoloration during drying, adverse changes in paper tonality and feel), these problems most likely are due to high-solution concentrations being employed. Even though conservators usually determine solution concentrations before treatment, there seems to be some uncertainty concerning the appropriate choice of concentrations. It appears that one objective still to be met in the discussion of paper washing is a definition of appropriate low-concentration bicarbonate solutions that, on the one hand, avoid potential adverse changes to paper and media, and on the other hand, offer an advantage over the use of calcium hydroxide solutions which can only be used at very low concentrations, due to the high alkalinity of the solution.
Post-Script
Large quantities of bicarbonate solutions are best prepared by using carbon dioxide delivered from a gas cylinder and bubbling it through a water suspension of magnesium (or calcium) hydroxide or magnesium (or calcium) carbonate. This, however, requires familiarity with the use of pressure reduction valves and enough space in a lab to house the equipment. for use in small workshop situations where only small volumes (five liter range) of dilute solutions are required, a seltzer bottle and disposable carbon dioxide cartridges have been recommended. We used a clear glass bottle with a protective metal mesh surrounding it for ease of viewing.7 Sparging one liter of water with one carbon dioxide cartridge dissolved about a half a gram of magnesium hydroxide or about a tenth of a gram of calcium hydroxide. the approximate solution concentration for calcium or magnesium bicarbonate can be determined with a color-indicator test kit8 or more exact concentration determination can be achieved by titration.
Acknowledgments
The authors wish to express their sincere gratitude to the paper conservators who kindly responded to the mail survey. Special thanks also go to Dr. F. Christopher Tahk for his invaluable advice concerning this project.
Notes
1. Burgess, H. B., and A.
Boronyak-Szaplonczay. 1992. "Uptake of Calcium or Magnesium into
Seven Papers During Aqueous Immersion in Calcium or Magnesium
Solutions." In Conference Papers Manchester 1992.
Leigh, United Kingdom: Institute of Paper Conservation. 264-72. Hey,
M. 1979. "The Washing and Aqueous Deacidification of Paper." the
Paper Conservator. Volume 4. 66-80.
2. Survey results were sent to
all paper conservators who responded to the questionnaire. All
comments and suggestions made by the respondents listed in the
results were kept anonymous.
3. Brederick, K., A.
Haberditzl, and A. Blüher. 1990 "Paper Deacidification in Large
Workshops: Effectiveness and Practicability." Restaurator 11.
165-178.
4. Wilson, W. K., R. Golding,
R. H. McClaren, and J. Gear. 1981. "The Effect of Magnesium
Bicarbonate Solutions on Various Papers." In Preservation of
Paper and Textiles of Historic and Artistic Value II, ed. J.
Williams. Washington, DC: American Chemical Society. 87-107.
5. Hey, M. 1981. "Stabilization
and Deacidification of Iron Gall Inks." Restaurator 5.
24-44.
6. The number eighteen
represents the total number of respondents who have used
bicarbonates (calcium bicarbonate and/or magnesium bicarbonate) in
the past and use them no longer. This number does not take into
account the type of material which they treated.