The Effects of Relative Humidity on Some Physical Properties of Modern Vellum: Implications for the Optimum Relative Humidity for the Display and Storage of Parchment
by Eric F. Hansen, Steve N. Lee, and Harry SobelAbstract
The effects of different relative humidities on some physical properties of three types of calf-skin parchment (vellum) were investigated. Standard samples were subjected to (1) tensile fracture and (2) measurement of the force that developed when the restrained samples were subjected to step decreases in relative humidity (RH) in the region between 60% and 11%. The results indicate that although no particular level of relative humidity can be excluded in general from consideration as a storage or display condition on the basis of tensile testing data alone, at 11% RH there is an adverse effect on some individual tensile properties. Relative humidities above 40% increase gelation and opportunities for biological growth. About 25% RH is the lowest level that can be tolerated without inducing large stresses in the material. On full consideration of these results, the physical chemistry and chemical reactivity of collagen, and the results of a recent study of the biodeterioration of parchment, a relative humidity of 30% seems optimum for such objects. At 30% RH, a cyclic variation of + 5% can be permitted with minimal effects of swelling and shrinkage.
Background
There are three primary concerns in the preservation of objects containing the protein collagen. First is the immediate or short term effects of the environment, primarily associated with temperature and relative humidity (RH), on the physical properties such as strength, flexibility, permeability and dimensional changes. Second is the long-term effects of the environment, associated with temperature, relative humidity, oxygen concentration, chemical pollutants and radiant energy (light), in causing "aging", or chemical modifications of the original material. Also important is how the environment allows biodeterioration by microorganisms and fungi of such a rich nutrient source.
The most commonly encountered recommendations in the conservation literature for the storage and display of parchment are in the region of 50% RH to 65% RH. However, recommendations vary and include unspecified levels such as "complete dryness" for the display of parchment and vellum: "the emphasis must be toward complete dryness with no excessive changes in temperature and humidity" (Bloodworth and Parkinson 1988). A recent review of the deterioration of skin in museum collections (Horde 1990) concludes that "Understanding of the complexities in preserved whole skin does not yet allow for adequate specification of 'ideal' conditions that will prevent deterioration." The discussion of an 'ideal' condition of relative humidity presented in this paper is a general one relating to a broad class of objects containing collagen as a principal component (such as parchment documents) and for which the conservation aim is the long term preservation of the collagen. It is presented with the understanding that there may also be other aims (relating either to aesthetic considerations or some use related function of a skin artifact) or other considerations (including the environmental requirements of other component materials of a composite object or the effects of additives) which must be considered in arriving at an "optimum" concentration of atmospheric moisture.
Some Properties of parchment. Parchment and leather are both processed from the skin of animals (vellum is a designation for parchment made from young or unborn calfskin). The history, techniques of manufacture and properties of parchment have been extensively reviewed by Reed (1975). In general, animal skins for both parchment and leather are dehaired through soaking in baths of various compositions and scraping of hair from the surface. At this stage, parchment manufacture differs from leather manufacture. The unique properties of parchment are due to drying the wet skin under tension; the tension causes an alignment of the three dimensional fiber network of the skin in a configuration more parallel to the surface of the skin. In contrast, the properties of leather are due to joining of the fiber network by chemical links introduced through tanning agents. The "leathered" skin excludes water from the fibers and is firmer and stronger as a result of tanning. Parchment, then, has a more aligned structure than leather, which has a complex, highly randomized weave structure.
Differences resulting from the two different processes, as noted by Reed (1975) are that:
- collagen fibers from parchment have a lower shrinkage temperature than collagen fibers from leather, (Collagen fibers exhibit a sudden shrinkage in length when heated. Fibers in leather have had chemical cross-links introduced which raise the temperature at which the fibers shrink (Haines 1987 ) )
- parchment soaks up water rapidly and in greater amounts than leather, and
- wet parchment has poorer resistance to microorganisms than leather, but greater resistance if kept dry.
Effect of atmospheric oxygen and water on collagen. Because parchment is processed from the skin of animals, its major constituent is insoluble collagen (which comprises about 95% of defatted, dehaired skin, with the percentage depending upon the age and sex of the animal). Therefore, the chemistry and structural stability of collagen at different levels of water content and resulting from different levels of relative humidity need to be considered.
As collagen ages, in vivo or in vitro, atmospheric oxygen plays a role in its chemical modification (Sober and Hansen 1989). Oxidation will change the molecular structure of the proteins, causing cross-linking or chemical modifications of several amino acids over time. Changes in parchment resulting from oxidation might also be influenced by the amount of residual fats and the type and amount of additives (such as softening agents) in historic parchment.
Water affects collagen in several different ways. Water plays a direct role in the chemical modification of collagen through hydrolysis, solvation of free radicals, hydrogen bond stability and the rate of gelatin formation.
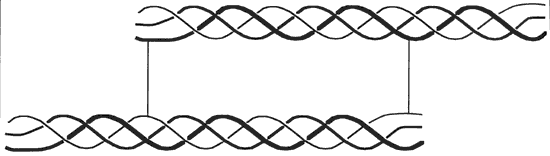
Figure 1. Triple helix of collagen (crosslinked to another molecule from peptides at end of molecule)
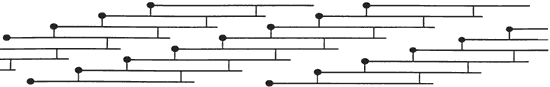
Figure 2. Collagen molecules line-up to form a fibril in "quarter staggered" array.
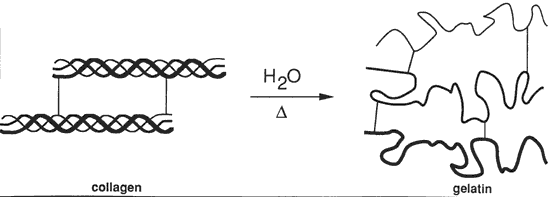
Figure 3. Denaturation of collagen
Collagen is composed of three similar strands of a protein containing in greatest amount glycine, alanine, proline, and hydroxyproline. The spatial configurations of repeated triple amino-acid sequences involving glycine, alanine and hydroxyproline or proline are responsible for the helical conformation between the strands. A triple strand of the protein is entwined in an alpha helix termed tropocollagen, shown in Figure 1 (from Scott 1986). Water molecules are intimately connected with the hydrogen bonding holding the triple helix together.
It is well known (Bull 1944, Dole and McLaren 1947) that water relates to collagen in four different processes, dependent upon the vapor pressure (relative humidity) shown in Table 1 (Koalov and Burdygina 1988). A definite amount of water is necessary for the molecular stability of the tropocollagen structure; this role for water is demonstrable in the region from <1% to 25% relative humidity. From approximately 25% to 60% RH the water is sorbed on hydrophilic sites in the proteins; above 60 % water is sorbed on polymolecular layers; and near saturation free water exists.
The tensile properties of collagen are a function of moisture content. Water sorption is also intimately involved in dimensional changes and swelling of the material.
Gelatinization of collagen. In addition to chain-breaking of a macromolecule by hydrolysis resulting in a decrease in molecular weight, another mode of protein degradation can occur through denaturation. In the case of collagen this is by interaction with water and is designated gelatinization, Figure 3. By heating collagen in water, the water molecules can gain sufficient energy to compete for the hydrogen bonds maintaining the triple helix configuration.
The rate of denaturation depends to some extent upon the intactness of the collagen fibrils that make up the fibers in skin. The tropocollagen molecules are cross-linked, end to end, and bundled together to form fibrils in the arrangement shown in Figure 2 (from Scott 1986). In skin, these fibrils form fibers which, in a three-dimensional array, are the primary component of skin. When the fibrillar structure is disturbed, as when the fibers are mechanically broken, collagen is more susceptible to attack by bacteria (Rebricova and Solovyova 1987).
Bowes and Raistrick (1967) studied the effect of relative humidity and pH upon the extent of hydrolytic breakdown of collagen and glutaraldehyde tanned collagen by determining the release of N-terminal residues. At 40°C for eight weeks, hydrolysis increased for both collagen and tanned collagen with increasing humidity (in the range from 40% to 100%) and decreasing pH (in the range from 5 to 2.5). They concluded that when hydrolytic breakdown becomes extensive, denaturation follows and this in turn accelerates further hydrolytic action.
Measuring the effect of temperature on the deterioration of collagen is hampered because temperatures at or above the shrinkage temperature may give unreliable results (Bowes 1964). Of particular interest is whether physically or chemically deteriorated collagen will deteriorate at a faster rate, but this has not been demonstrated and is a matter for further study. What is known is that denaturation of disrupted collagen is promoted by less extreme conditions than those which initiate denaturation in intact collagen. The physical and chemical integrity of collagen can be indicated by the hydrothermal stability, as shown by a decrease in the shrinkage temperature for deteriorated fibrils (Young 1990).
Introduction
This paper is a discussion of the factors to be considered in determining an optimum level of relative humidity (RH) for the display and storage of parchment and other skin artifacts, based upon both a consideration of the physical chemistry and chemical reactivity of collagen and upon physical tests of modern vellum over a range of relative humidities. The Charters of Freedom of the United States (the Constitution and Declaration of Independence) and the Dead Sea Scrolls will be used as examples for this discussion. Indeed, the arguments presented for the optimum level of relative humidity for display and storage, 30% RH, do not differ significantly from those proposed by the National Bureau of Standards for the Charters of Freedom in 1951 (National Bureau of Standards 1951). The effects of light and concentrations of air pollutants on the deterioration of collagen, although important factors which are in all probability also dependent upon the concentration of oxygen and atmospheric moisture, are not included in the scope of this discussion.
In a recent review of storage conditions for archives and libraries, Wilson (1986) noted that conditions for parchment and vellum could not be discussed in as concise a way as for leather and paper because systematic data on the physical properties of parchment and the degradation rates of parchment over a wide range of relative humidities were not available. In an effort to alleviate this situation, data were acquired and are reported here for the following physical properties of three types of modern vellum over a range of relative humidities from 11% to 60%:
- tensile properties of equilibrated samples fractured at a constant rate of elongation, and
- a measure of the force developing in a restrained sample that is exposed to successively lower relative humidities.
Experimental
Materials. As previously discussed, the distinct properties of parchment are a result of the production process, which includes drying under tension. Some "parchment" sold today is not true parchment, but a formaldehyde tanned skin. Commercial parchment may be true parchment, but may include additives and softening agents. The parchment used in this study was purchased from a vendor of true parchment. The general procedure for making parchment has been previously described (Vorst 1984).
Three types of vellum were purchased (the definition of vellum used here is one of parchment made from young calf) and labeled for convenience in distinction as "Modern", "Medieval", and "Talmudic." These designations do not indicate that the tensile properties of the vellum designated "Medieval" compare with the tensile properties of parchment manufactured during the Medieval period, but rather that a particular aspect of the production process was held in common with the Medieval practice of parchment making. Thus, the vellum labeled "Medieval" was more fully neutralized with rinsing and soaking in comparison to the vellum designated "Modern." The vellum designated "Talmudic" was, in addition to being neutralized fully with ammonium chloride, also subjected to a bath that contained small amounts of oak gall. The designation "Talmudic" was chosen because the ancient Hebraic method for the production of parchment either included a tanning agent in a bath or the surface was subsequently finished with a tanning agent, as shown by analysis of Dead Sea Scroll samples (Poole and Reed 1962).
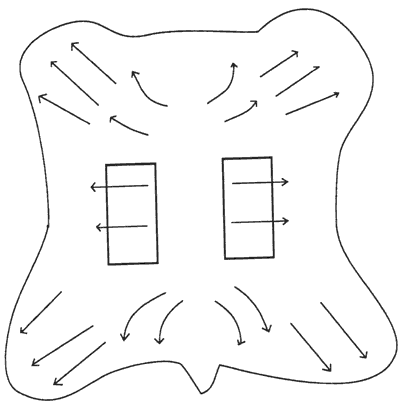
Figure 4. Approximate directionality of collagen fiber bundles and area of skin sampled.
Sample cutting and conditioning. The test samples were cut from selected areas of the whole skin as depicted in Figure 4 (from The Leather Conservation Center, 1981), in an attempt to obtain samples with maximum similarity in the direction of the collagen fiber bundles. Dogbone shaped samples were cut in accordance to ASTM test method D 2209-80 (ASTM 1989), "Standard Test Method for Tensile Strength of Leather." However, due to the size of the tensile testing equipment the sample dimensions were scaled down by a factor of 2 reducing the testing width from 12.5 mm to 6.3 mm. The thickness of the Talmudic type vellum samples was 0.2 + 0.01 mm, of the Medieval type vellum samples was 0.2 + 0.03 mm, and the Modern type vellum was 0.23 + 0.03 mm.
All dogbone shaped samples were preconditioned in excess of three months prior to testing in a humidity chamber at either 43% RH, 33% RH, 22% RH or 11% RH maintained by saturated salt solutions of potassium carbonate, magnesium chloride, potassium acetate or lithium chloride, respectively. These salt solutions were chosen for their vapor pressure, which results in the desired relative humidity in an enclosed chamber at 22°C. The samples conditioned at 60% RH were not placed in a humidity chamber but were allowed to be conditioned in a temperature and humidity controlled room where the relative humidity fluctuated at 60 + 5% and the temperature was also at 22 °C.
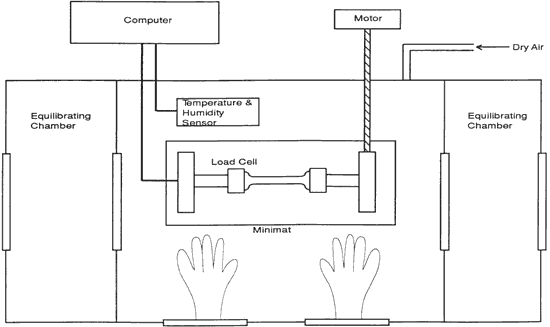
Figure 5. Diagram of tensile testing chamber. Samples can be introduced from a side cabinet without exposure to higher relative humidities.
Tensile properties. Tensile testing and the measurement of the stress in restrained samples were performed on a Polymer Laboratories Minimat tensile testing instrument placed inside a micro-environment chamber built at the Getty Conservation Institute (See Figure 5). It was necessary to have a chamber with interlocking side-chambers so that samples and salt solutions could be introduced without exposing the central chamber to the ambient relative humidity of the room, which is 60% RH. The vellum samples sorb water very quickly when exposed to an increased level of relative humidity.
The humidity in the chamber during testing was maintained with the same saturated salt solutions used for conditioning the samples. The gauge length was 0.050 m and the constant rate of extension was 0.020 m/mint Ten replicate samples of each of the three vellum skins were tested on the Minimat at each relative humidity. (These ten samples were randomized to include samples from opposite sides of the skin in each set.) Test data were recorded on an IBM AT computer with a data acquisition program that utilizes an external I/O board that converts analog signals to digital signals.
Temperature and humidity were simultaneously recorded on the computer using a General Eastern Humidity and Temperature Sensor.
Collected data were saved for further analysis on the Apple MacIntosh FXII computer using Stat View and Cricket Graph software. Because of the more convenient use of a larger tensile testing instrument, tensile fracture for the samples conditioned at 60% RH was performed with an Instron Model 4201 Universal Testing Instrument in the same temperature and humidity controlled room. Tensile test data were stored for reanalysis using Instron software, "General Tensile Test, Revision D".
Force developing at a fixed gauge length. For the humidity downramping tests, straight edged, 0.0127 m wide samples were cut with a Thwing Albert precision sample cutter and from the same areas of the skins as for tensile testing. The gauge length was 0.050 m and the samples were held at a 2 N pre-load in the clamps of the tensile tester. The chamber and the sample were then allowed to equilibrate at an initial relative humidity of 60%. After equ ilibration, the humidity was lowered to 43% by an influx of dry air, and then this humidity was maintained with the appropriate saturated salt solution while the sample was allowed to equilibrate. (The relative humidity was decreased in a step-wise manner, rather than having the humidity reduced by equilibration with the specific salt solutions). The chamber conditions and the developing tension were monitored with a General Eastern Temperature and Humidity Transmitter and the Minimat, respectively. Data from both instruments were recorded using an IBM AT computer and an I/O board. Data were collected at five minute intervals for a duration of several days; the required time was dependent on the rate at which equilibration was achieved at 43%, 33%, 22%, and 11% RH. Equilibration was assumed when the force that developed held constant for 24 furs. Each type of parchment was subjected to this procedure.
Results and Discussion
Results of tensile testing. The properties of the vellum used in this testing should reflect those of vellum that has not been treated with softening agents or other additives.
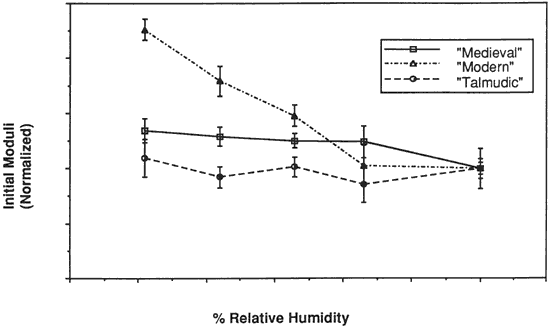
Figure 6. Initial moduli of Tree Vellum Types at Various % Relative Humidities
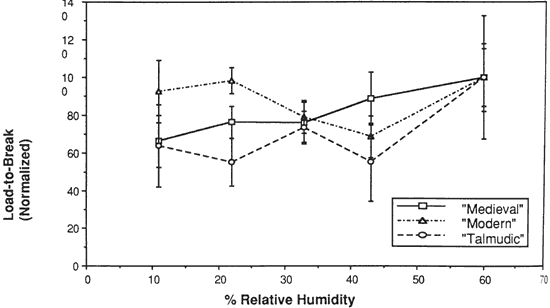
Figure 7. Load-to-Break of Three Vellum Types at Various % Relative Humidity
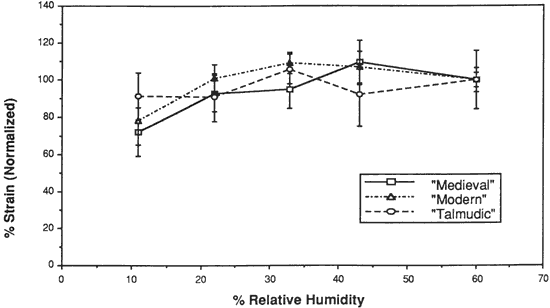
Figure 8. % Strain-to-Break of Three Vellum Types at Various % Relative Humidity
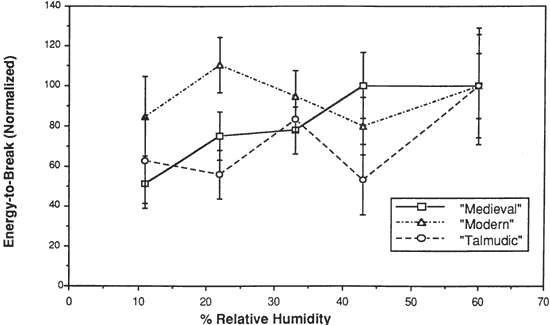
Figure 9. Energy-to-Break of Three Vellum Types at Various % Relative Humidity
The initial modulus, load-to-break, percent strain-to-break and energy-to-break (tensile energy absorption) obtained from tensile fracture after equilibration at different relative humidities are tabulated in Tables 2, 3, 4 and 5, respectively. The same properties, normalized to a value of 100 for a tensile property of each type of vellum at 60% RH, are shown in Figures 6, 7, 8, and 9. The tensile properties were normalized to more clearly illustrate a comparison of the relative change for each type of vellum. (As the response of the humidity sensor is not linear below 20% RH, the recorded value was 15% over a saturated lithium chloride solution. Since the recognized RH of a saturated lithium chloride solution is 11%, this value is used in the charts and graphs.)
The initial modulus determined for Modern vellum is increased to the greatest extent as a result of equilibration at lower relative humidities, with Medieval vellum showing a similar trend. The value of the initial modulus for Talmudic parchment is relatively unaffected.
The effect of changes in relative humidity on the load-to-break mean obtained after equilibration and fracture is greatest for the Medieval vellum, with a reduction in the load-to-break mean for each reduction in RH over the range from 60% to 11%, and with the value at the lowest RH significantly different from the value at the highest RH. In contrast, Talmudic vellum has relatively static values in the region from 43% to 11% RH, with a reduction in the loads-to-break mean only upon lowering the humidity from 60 % to 43% RH. The Modern vellum exhibits no statistically significant reduction in breaking load upon lowering the RH, i.e. the values obtained at each level of RH are within the range of the standard deviations.
There is no significant change in the percent strain-to-break at different relative humidities for Talmudic parchment. However, while the values for both Modern and Medieval types remain static from 60% to 22% RH, there is a clear reduction in percent strain-to-break upon reducing the RH below 22% to 11%.
The energy-to-break is calculated from the area under the stress-strain curves. Because the energy required to fracture a sample reflects changes in both the ability to elongate and also to bear a load, this quantity is most representative of the tendency of parchment to fracture at a certain level of relative humidity. However, this quantity also has a wider spread in the standard deviation because both the uncertainty in the strain-to-break and load-to-break are represented. For both vellum types designated Modern and Talmudic, decreasing the relative humidity from 60% to 11% does not result in a mean value outside the uncertainty (standard deviation) of the values at 60%. For Medieval parchment, there is a clear reduction in the mean energy-to-break to half (50%) the original value.
Does the effect of lowering the relative humidity increase the "brittleness" of newly manufactured vellum? To answer this question, the difference between "brittleness" and "stiffness" should be defined. A "stiffer" material has a higher value for the initial modulus (an indication of inelastic deformation), and therefore the vellum prepared in the Modern method became stiffer with a decrease in humidity. "Brittleness," however, is a reduction in the ability to elongate before breaking. Therefore both the Medieval and Modern vellum can be considered more brittle at lower relative humidity levels.
These results pertain to two questions:
- are the tensile properties largely affected at humidity levels in this range; and,
- why do these data differ from the frequent observation that parchment under "dry" conditions becomes brittle? For example, Werner states that "parchment documents...kept under conditions that are too dry, say at 45 percent relative humidity or lower...tend to cockle and to become rather rigid" (Werner 1968).
Figure 10.
Figure 11.
Figure 12.
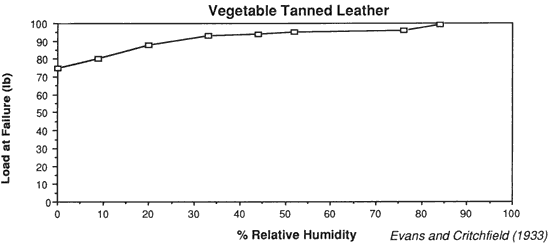
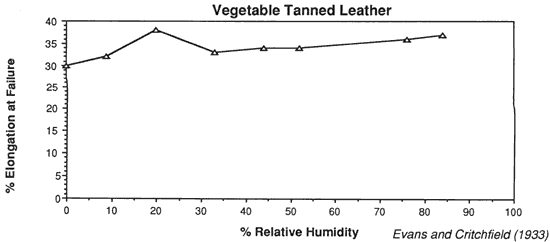
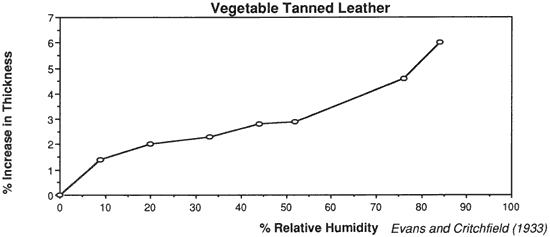
Wilson (1986), in reviewing storage conditions for leather in archives and libraries, considered the results of testing the tensile properties and dimensional changes of vegetable tanned leather by Evans and Critchfield (1933), reproduced in Figures 10 through 12. He concluded that the changes were not large enough to preclude the storage of vegetable tanned leather at any of these relative humidities on the basis of this data alone. The same general conclusions can be reached for the results of tensile testing on vellum presented here, except for the fact that at 11% RH a notable reduction is evident in some individual properties of specific vellum types: the load-to-break of Medieval vellum; the strain-to-break of Medieval and Modern designated vellum; and the energy-to-break of the vellum designated Talmudic.
Several effects may account for observations other than those presented here that some parchment becomes brittle below 43% RH. As originally produced, skin artifacts should vary in tensile properties for a variety of reasons including the age at which the animal was slaughtered, the species of the animal, the health of the animal, the sex of the animal and, being a natural product, the individual variations between two animals of the same species. In addition, the variety of procedures used in manufacture (length of time in water-bath, chemicals used, effectiveness of fat removal, amount of tension applied in parchment making) may affect the physical state of the material. With either increased age or increased exposure to environmental extremes, there may be more extensive oxidation of proteins, oxygen induced cross-linking, increased gelatinization and an increase in lower molecular weight fractions due to hydrolysis. These modifications may affect the tensile properties of the parchment at differing moisture content. Extensive chain-scission and denaturation has been associated with extreme brittleness of parchment (Horde 1990).
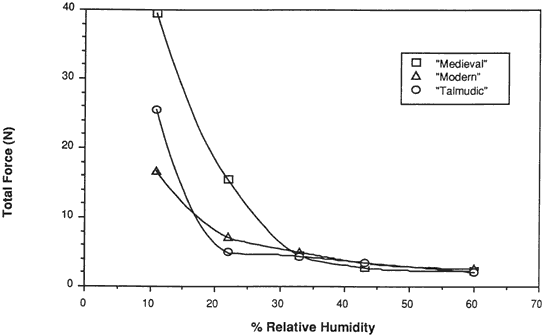
Figure 13. Restrained Force Development of Parchment on Lowering of Relative Humidity
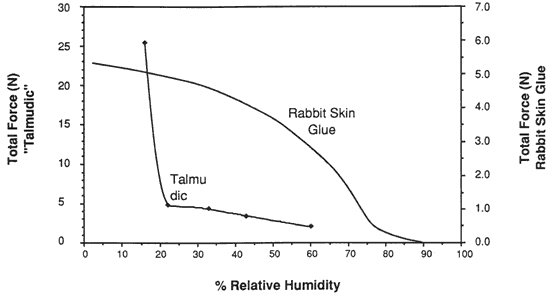
Figure 14. Restrained Force Development of Cast Rabbit Sking Glue (Gelatin) on Lowering of Relative Humidity
Results of testing restrained samples. The same relative behavior was observed for all three types of vellum when restrained at a fixed gauge length and the relative humidity was lowered. As the humidity was lowered from 60% to 22% relatively little force developed in a restrained sample. However, as the water content was decreased below that contained in vellum equilibrated at 22% RH, there was a significantly large increase in the force (see Figure 13). These results clearly indicate that around 25% is the lowest level that can be tolerated without inducing large forces in the vellum.
The last result may be explained in reference to the four regions of water sorption associated with the collagen molecule. Below 25% RH water is removed from the tropocollagen structure, causing an unstable chemical state and, as indicated by the forces developing upon shrinkage, an undesirable mechanical state.
A comparison of the force developing as the relative humidity is lowered in a restrained sample of vellum (with a high portion of intact collagen) with the force developing in a sample of cast rabbit skin glue (a highly gelatinized material) is shown in Figure 14 (Data from Mecklenburg 1988). A completely different profile, noticeably lacking a large increase in stress below 22%, results in the plot of force development versus relative humidity in the case of rabbit skin glue, wherein little intact tropocollagen is present.
Implications for storage conditions. As has been shown, there is a minimum level of relative humidity that should be maintained; storage of a parchment below 25% RH is not indicated. Above this relative humidity all strongly water sorbing groups on collagen should be saturated. A similar position was taken by the National Bureau of Standards when the display and storage conditions for the Charters of Freedom of the United States (Declaration of Independence and the Constitution) were defined in 1951 (NBS 1951). These parchment documents are stored under an inert atmosphere of helium humidified to 25% relative humidity. The reasoning of the NBS was based on two sources:
- a study conducted at the NBS on the effects of temperature and oxygen on the deterioration of leather (Kanagy, 1940) which indicated that the rate of deterioration increases with temperature and that the rate is greater in an oxygen atmosphere than a helium atmosphere; and,
- on the sorption curves of collagen at 37°C (Kanagy 1947).
Several issues can be raised about the advisability of the long term storage or display of parchment below the current recommendations for relative humidity which are usually 50%.
- Possible complications may occur because of the composite nature of a document. Parchment objects may be pigmented on the surface, as in the case of illuminated manuscripts, or may have been written with an ink that is not well bound to the surface. Physical loss of paint or ink may occur with a loss of water content or because of a difference in dimensional changes in the two materials (vellum and colorants) with changes in relative humidity.
- Rehydration of fully desiccated collagen does not occur with humidification. However, considering the theoretical model of the response of collagen to relative humidity, an exposure to a relative humidity level of 30% will not result in this type of desiccation, removing only water involved in side chains. On the basis of this reasoning, the effect on the physical properties of long-term storage would be reversible with an increase in humidity. One would like to see confirmation from a long term storage study.
- A reasonable concern might be that the molecular chains are closer together under drier conditions. The question of accessibility to oxidative cross-linking should be studied.
- If the material is quite brittle at a lower relative humidity and subject to manipulation, more damage is likely to result.
- Parchment may buckle or curl at lower humidities, a condition which may not necessarily be physically damaging but may be aesthetically objectionable.
- Although not particularly applicable in the consideration of the Charters of Freedom where considerable funds are available for environmental control, a lower relative humidity (30%) is in some instances more difficult to maintain without cycling than 50%. Cycling of humidity (due to fluctuations of temperature and ambient humidity) is of particular concern because as a result of the swelling and shrinkage of the material mechanical damage may eventually occur, which will result in the case of collagen with a material more easily susceptible to the negative effects of water and oxygen.
The negative effects of cyclic conditions of wetness and dryness on leather (Witnauer and Palm, 1968) has been demonstrated by measuring the hydrothermic stability (the shrinkage temperature). After two months of cycling, the shrinkage temperature of a chromechrome glutaraldehyde retan tanned leather was reduced 3°C when exposed to cycling between 3 days at 50 % RH and 4 days at 72% RH at 23°C. The shrinkage temperature was reduced 7°C when exposed to cycling between 18 hours under vacuum and 6 days at 50% RH.
However, there are also several issues that should be raised about the advisability of a 50% relative humidity level for the display and storage of ancient and culturally important parchment documents. The Dead Sea Scrolls are an example of parchment documents whose condition (present state of deterioration) demands further consideration.
The Dead Sea Scrolls are a collection of over 500 parchment documents, mostly in incomplete states. The first documents were found previously to 1947. Their manufacture has been attributed to the Qumran community (a particular Hebraic sect) and roughly dated between 200 B.C. and 100 A.D. The technology used in their manufacture differs from other parchment manuscripts in that lime was probably not used in the dehairing bath and in the presence of surface tannage (Poole and Reed 1962).
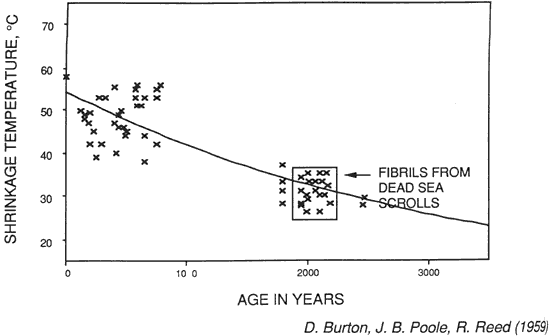
Figure 15. Dating of Dead Sea Scrolls on Archaelogical and Palaeographic Grounds
In attempting to date the Scrolls, Burton, Poole and Reed (1959) compared the shrinkage temperature of collagen fibers in the scroll fragments to the shrinkage temperature of samples from parchment or raw hide of known origin (between 1000 B.C. and 1000 A.D.). They reasoned that degenerative changes in the collagen fibers would be due to those that occur with the passage of time. The results, shown in Figure 15, show that the shrinkage temperature of collagen from the Scrolls, like other older collagen, is low (between 22 and 35°C) and consistent with the probable time of origin.
Other studies have shown that much of the collagen is not intact. Weiner, Kustanovich, Gil-Av and Traub (1980) examined small pieces of scroll material and random, unwritten fragments in order to determine the collagen to gelatin ratio using X-ray diffraction and amino-acid racemization analyses. By their X-ray diffraction method, they determined the collagen to gelatin indices (C:G) of modern rat-tail collagen to be 10:2, and the C:G of gelatinized rat-tail collagen to gelatin to be 0:7. C:G indices of modern parchment samples were 9:9, 9:8 and 12:5. The C:G indices of 34 samples from scrolls or fragments ranged from 0.6 to 6.0, indicating a wide range and degree of denaturation in the samples.
Samples of scroll fragments were recently analyzed at the Getty Conservation Institute (Derrick 1992) by Fourier transform infrared spectroscopy (FT-IR) using both a surface technique (Attenuated Total Reflection) and an FT-IR microscope to scan cross-sections. This method can detect increased denaturation by a shifting of an absorption band to a lower frequency. The amide II band shifts from 1550 to 1530 cm-1 when the collagen helix is converted to gelatin (Brodksy-Doyle, Bendit and Blout 1975; Susi, Ard and Carroll 1971). Differences in chemical composition resulting from hydrolysis or oxidation can be detected by spectral comparison of modern parchment samples and samples from the Scrolls. Analysis indicated evidence of denaturation, hydrolysis and oxidation which were associated to a greater extent with both brittle areas and also darker, discolored areas.
Further analysis included amino acid analysis of modern parchment and scroll parchment by High Performance Liquid Chromatography. Modern parchment was 90% or more extractable by weight, when extracted for collagen, in the preparatory acid hydrolysis prior to amino acid analysis. In contrast, scrolls fragments were less than 70% extractable by weight, indicating extensive oxidative modification of amino acids may have occurred (Sober 1990).
The Dead Sea Scrolls, then, exhibit a state of deterioration consistent with their age. The extent of degradation is varied, but includes evidence of hydrolysis and oxidation in addition to denaturation. Problems which exist with a 50% recommended relative humidity for parchment documents are:
- Biodeterioration has been shown, for some aerobic and anaerobic microorganisms common to parchment deterioration, to be dependent upon relative humidity levels (Valentin, Lindstrom and Preusser 1990). Growth was active above 40% RH. Below 40% RH, growth could not be detected with the radioactive tracer method used in their study.
- Samples of the Dead Sea Scrolls have been shown by analysis to have deteriorated to some extent.
Of particular concern is the shrinkage temperature, especially the lower limit found to be 22 °C. As noted in the introduction, denaturation and hydrolysis occur more easily when the collagen has lost physical or chemical integrity.
The Dead Sea Scrolls are of particular interest and cultural importance, being among the earliest extant Biblical documents. Factors affecting the long term preservation of these documents (relative humidities lower than 50%) outweigh immediate aesthetic effects, such as possible curling or curvature of a document. Concerns about negative aesthetic effects which result from decreasing the water content in parchment within a certain regime must also be viewed with the understanding that these effects may be reduced. Lowering the rate of drying may lessen the effect of lowering the water content. A document could also be constrained in a particular shape, but this might also lead to cracking.
The above considerations indicate that the practice of humidifying brittle historical parchment at 100% RH to relax the material may be a questionable one. Exposure to a high relative humidity may have the transitory, beneficial effect of relaxing the parchment to allow unrolling or other manipulations. However, the chemical state of the material, both immediately after relaxation and in the future, need to be more fully determined regarding possible adverse affects.
Conclusion
This study has sought the answer to the question of what is the optimum relative humidity for objects containing collagen as a principle component. Decreasing the water content reduces both the possibility of biodeterioration and the probability of future environmentally induced chemical modifications of collagen. A lower limit has been discussed for collagen; storage or display below around 25% RH is not indicated. Because of possible cycling effects of plus or minus 5%, a slightly higher value, 30% RH, is suggested as the optimum condition for an object where the long-term preservation of the intact collagen is of greatest concern.
However, there are other considerations, particularly in regard to the degree to which a skin object must be manipulated or in determining the possibility of the loss of inks or colorants. In this regard, determining the optimum relative humidity for remains derived from skin has some parallel in the history of conservation in relation to determining safe-levels of illumination. When determining the optimum light levels for the display of light-sensitive objects, the illumination recommended is the lowest that will allow the required visibility. In the case of remains derived from skin, the lowest amount of atmospheric moisture (above 25% RH) that will allow the mechanical requirements, the consideration of other composite elements and the aesthetic requirements is indicated.
Previous discussions of optimum relative humidity levels for parchment which have recommended 50% RH or higher have focused primarily upon the pliability and other physical properties of parchment. The work just reported has shown that for organic materials, such as proteins, a knowledge of the chemical changes which occur over time may be as important as a knowledge of the physical properties in determining the most reasonable display and storage conditions.
It can be stated that the conditions for maintenance of collagen containing objects should be geared either for the intent of its exhibition or usage (long-term display or short-term handling), keeping in mind that relative humidities below 25% are incompatible with long term maintenance as are relative humidities above 40%, which increase hydrogen bond breakage, gelatinization and biological growth.
Acknowledgements
The contents of this paper have been submitted to the Journal of the American Institute of Conservation.
The authors would like to thank Neville Agnew, Mary Chase, Michele Derrick, Robert Feller, Marylou E. Florian, William Ginell, Betty Haines, and David Scott for their comments and suggestions; Carol Sussman for her assistance in preparing this manuscript; Andrew Kim and Raphael Garcia for assistance in the laboratory; Jack Gromek for software support in data collection and analysis; and Jim Davies for assistance in manufacturing the testing chamber.
References
American Society for Testing Materials. 1984. 1984 Annual Book of ASTM Standards. Philadelphia: American Society of Testing Materials:15.04:453-455.
Bloodworth, J. G. and Parkinson, M. J. 1988. The display of parchment and vellum. Journal of the Society of Archivists. 9(2):65-58.
Bowes, J. H. and Raistrick, A. S. 1964. The action of heat and moisture on leather. Part V. Chemical changes in collagen and tanned collagen. The Journal of American Leather Chemists Association. 59:201-15.
Bowes, J. H. and Raistrick, A. S. 1967. The action of heat and moisture on leather. Part VI. Degradation of the collagen. Journal of American Leather Chemists Association. 62:240-57.
Brodsky-Doyle, B., Bendit, E. G. and Blout, E. R. 1975. Infrared spectroscopy of collagen and collagen-like polypeptides. Biopolymers. 14:937-957.
Bull, H. B. 1944. Adsorption of water vapor by proteins. Journal of American Chemical Society. 66:1499-1507.
Burton, D. M.B.E., Poole, J. B. and Reed, R. 1959. A new approach to the dating of the Dead Sea Scrolls. Nature. 164:533-534.
Calmes, A. 1985. Charters of Freedom of the United States. Museum. 146:99-101.
Derrick, M. 1992. Evaluation of the state of degradation of Dead Sea Scroll samples using FT-IR spectroscopy. Annual of the Book and Paper Group: Papers Presented in the BPG Specialty Group Session at the 18th Annual Meeting of the AIC. Albuquerque. New Mexico (in press).
Dole, M. and McLaren A. D. 1947. The free energy, heat and entropy of sorption of water vapor by proteins and high polymers. Journal of the American Chemical Society. 96:651-657.
Evans, W. D. and Critchfield, C. L. 1933. The effects of atmospheric moisture on the physical properties of vegetable and chrome tanned calf leathers. Journal of Research of the National Bureau of Standards. 11:147.
Haines, B. M. 1987. Shrinkage temperature in collagen fibres. Leather Conservation News. 3:2:1-5.
Horie, C. V. 1990. Deterioration of skin in museum collections. Polymer Degradation and Stability. 29:109-133.
Kanagy, J. R. 1940. Effect of oxygen concentration and moisture on the stability of leather at elevated temperatures. Journal of Research. National Bureau of Standards 25:149.
Kanagy, J. R. 1947. The adsorption of water vapor by untanned hide and various leathers at 100? F. Journal of American Leather Chemists Association. 42:98.
Kozlov, P. V. and Burdygina, G. I. 1983. The structure and properties of solid gelatin and the principles of their modification. Polymer. 24:651-666.
Leather Conservation Center. 1981. The Fiber Structure of Leather. London: Leather Conservation Center.
Mecklenburg, M. F. 1988. The effects of atmospheric moisture on the mechanical properties of collagen under equilibrium conditions. Preprints of the American Institute of Conservation 16th Annual Meeting. New Orleans, Louisiana. June 1-5. 1988 ed. Rosenberg, S. Z. (Washington, D.C.: American Institute for the Conservation of Historic and Artistic Works) 231-242.
National Bureau of Standards. 1951. Preservation of the Declaration of Independence and the Constitution of the United States, National Bureau of Standards Circular 505.
Poole, J. B. and Reed, R. 1962. "The preparation of leather and parchment by the dead sea scrolls community", in Technology and Culture. 1-26.
Rebricova, N. L. and Solovyova, N. I. 1987. "Electron microscopic and biochemical investigation of parchment", in ICOM Committee for Conservation, 8th Triennial Meeting. Sydney.
Reed, R. 1975. The Nature and Making of Parchment, England: The Elmete Press.
Scott, J. 1986. "Molecules that keep you in shape", in New Scientist. 49-53.
Sobel, H. 1990. Private communication. Isotope Laboratory, Institute of Geoplanetary and Planetary Physics, University of California, Los Angeles, 90024.
Sobel, H. and Hansen, E. 1989. Environmentally produced changes in historic proteins: A. Collagen. Text of poster presented at The Third Annual Symposium of the Protein Society, Seattle, Washington, August, 1989.
Susi, J., Ard, J. S. and Carroll, R. J. 1971. "Hydration and denaturation of collagen as observed by infrared spectroscopy", in Journal of American Leather Chemists Association, 66(11):508-519.
Valentin, N., Lindstorm, M. and Preusser, F. 1990. "Microbial control by low oxygen and low relative humidity environment", in Studies in Conservation, 35:222-230.
Vorst, B. 1986. "Parchment making - ancient and modern", in Fine Print. 12(4):209-211,220-222.
Weiner, S., Kustanovich, Z., Gil-Av, E. and Traub, W. 1980. "Dead Sea Scroll parchments: unfolding of the collagen molecules and racemization of aspartic acid", Nature, 287:820-824.
Werner, A. E. 1968. "The conservation of leather, wood, bone and ivory, and archival materials", in The Conservation of Cultural Property with Special Reference to Tropical Conditions. pp. 265-290.
Wilson, W. K. 1986. Guidelines for Environmental Conditions for Storage of Paper-Based Non-Photographic Records in Archives and Libraries (unpublished manuscript). 1401 Kurtz Road, McLean, Virginia, 22101.
Witnauer, L. P. and Palm, W. E. 1968. "Influence of cyclic conditioning on the hydrothermal stability of leather", Journal of American Leather Chemists Association. 63:333-345.
Young, G. S. 1990. "Microscopical hydrothermal stability measurements of skin and semi-tanned leather, in ICOM Committee for Conservation 8th Triennial Meeting. Dresden. 19(2); 626-631.
Eric F. HansenGetty Conservation Institute
Steve N. Lee
Getty Conservation Institute
Harry Sobel
Isotope Laboratory, Institute of Geoplanetary and Planetary Physics
University of California
Publication History
Received: Fall 1991
Paper delivered at the Book and Paper specialty group session, AIC 19th Annual Meeting, June 3-8, 1991, Albuquerque, New Mexico.
Papers for the specialty group session are selected by committee, based on abstracts and there has been no further peer review. Papers are received by the compiler in the Fall following the meeting and the author is welcome to make revisions, minor or major.