Investigation of Some 12th Century Chinese Papers
by Harald BerndtAbstract
A conservator presented with the challenge of treating five 12th century Chinese documents approached the Wood Artifacts Conservation Group at the University of California Forest Products Laboratory for help in classifying a granular material covering most of the documents. The granules were examined using infrared spectroscopy and were found to be an organic material. After subsequent research and discussion with the conservator we concluded that it was a polysaccharide similar to gum arabic. The conservator subsequently incorporated our findings into the treatment plan.
Introduction
This investigation was carried out to address a specific problem faced by a conservator. Providing an answer involved chemical analysis, literature search, and discussions with conservators and other scientists.
The object of concern was a set of five documents found in the relic chamber of a Chinese Buddhist statue that had been donated to the New Orleans Museum of Art. Two of the documents were dated 1155 and 1174, respectively (Wood 1986). The late Keiko Keyes was asked to perform a conservation treatment on the documents. She involved Linda Ogden in the project, who carried out the actual treatment (Ogden 1990).
This report will describe the problems that prompted Keiko Keyes to seek scientific advice, the conclusions we cooperatively arrived at and their effects on a resultant treatment plan. Since the treatment is now completed (Ogden 1991), this paper also briefly reports the results.
Description of the Documents and Their Condition
According to Wood (1986), the documents
"... consist of three sutras, ..., fragments of what appears to be a history of the transmission of Buddhism from India to China, and a slip of paper that is possibly the original inventory list of objects placed in the statue."
The documents were printed with woodblock in black ink on linen paper. The three sutra texts were accordion-fold books with 9 to 18 leaves, measuring between 251 mm and 290 mm in height and between 72 mm and 92 mm in width. The paper of the oldest, dated 1155, was tinted yellow, apparently with a natural insecticide produced from Phellodendron amurense (Keyes 1989 A).
All documents were severely soiled throughout, with deposits of fine brownish dust, straw and other debris. The paper was basically in good shape though it felt slightly brittle. There was some mechanical damage around the edges, and the folds were broken in some places. Its pH was 5.0 to 5.5. It showed tide stains and local discolorations, and the colorant was mottled and streaked due to contact with excessive moisture (Keyes 1989).
Figure 1. Photomicrograph of chinese paper showing granular material and linen fibers.
The specific concern prompting more thorough analysis was the dense deposit of granular material throughout the papers, particularly the dated sutras. To the naked eye, the granules appeared to be of different color and structure in different regions of the papers, but stereo-microscopic examination revealed more similarities than differences. In several places, this deposit appeared starkly white, especially prominent on top of the black ink (Figure 1). This appearance suggested that it might be a potentially aggressive salt that could have dissolved during aqueous treatments and caused damage to the paper.
Analysis of the Paper and the Adhering Granular Material
Figure 2. Diffuse Reflectance infrared (DRIFT) spectra of three historic linen papers and Whatman No. 1 filter paper
(Figure 2 shows the diffuse reflectance infrared (DRIFT) spectra of one of the document papers and several other papers. Comparison with the spectrum of Whatman No. 1 filter paper shows only one significant difference. All linen paper spectra, including that of a contemporary sample not shown in (Figure 2, have a peak near 1650 cm-1. According to the literature (Liang et. al. 1960), this is due to adsorbed water. Water absorbs infrared radiation strongly, wherefore samples are dried before analysis. In this experiment, the paper samples were stored in the sample chamber of the infrared spectrometer, which is continuously flushed with a stream of dry air, for 24 hours prior to analysis. This treatment sufficed to dry the Whatman No. 1 filter paper completely, but apparently did not remove all bound water from the linen papers. The remaining water in the linen papers must be either bound more strongly to the cellulose surface or trapped in isolated microcavities in the cell walls. Both explanations point to physical differences between the celluloses in the two types of paper.
The spectroscopic evidence is not strong enough to rule out chemical degradation of the manuscript paper, as depolymerization via acid hydrolysis would not change the spectrum significantly. Similarly, mild oxidative degradation may not show. On the other hand, a pH of 5.0 to 5.5 does not necessarily indicate any damage. Pure alpha-cellulose was reported to have a pH of 5.0 to 5.3 (Stamm 1964).
Visual examination of the document paper using a stereo microscope showed that the granular material conspicuous on the surface was also present in deeper layers of the paper ((Figure 1). Attempts to remove some of the granules with a preparation needle showed that they adhered strongly to the fibers.
As much as possible of the granular material from the available sample was separated under the stereo microscope, collected on a microscope slide and used to prepare potassium bromide (KBr) micropellets for use in Fourier transform infrared spectroscopic (FTIR) analysis. The resulting spectrum indicated an organic material, probably a polysaccharide.
Keiko Keyes received the visual and spectroscopic evidence indicating that the material in question was probably a polysaccharide and an integral part of the paper with a request for information about materials used in the papermaking processes of the period and region. A literature reference to Japanese papermaking she supplied (Hughes 1978) showed that mucilages (vegetable gums) were added to the papermaker's vats.
Infrared spectra of unknown granular material, gum arabic and gum tragacanth
This information prompted a literature search for infrared spectra of gums. A publication by colleagues from the Conservation Analytical Laboratory of the Smithsonian Institution (Erhard et. al. 1988) included a spectrum of gum tragacanth, which had features similar to the spectrum of the unknown material. To make a proper comparison, authentic samples of gum arabic and gum tragacanth were obtained (Burke 1989). These were prepared for infrared analysis in the same way as the sample of the unknown. (Figure 3 shows the resulting three spectra. It can be seen that the overall features are very similar, and most peaks can be matched up one-to-one for the unknown and gum arable.
A solubility/swelling test of the granular material was carried out informally by wetting some on the microscope slide used for its collection and visually observing any changes over several weeks. The granules did not appear to interact at all with the water and showed no discernible swelling or softening.
Interpretation of the Analysis and Its Use for the Treatment Plan
Infrared analysis is an empirical technique, depending entirely on comparisons between substances of known composition. Also, details of the physical state of the sample, as affected by its history and the sample preparation, may change the appearance of infrared spectra significantly. Thus, it is very difficult to unequivocally identify an unknown compound with unknown history by infrared analysis alone. Similarly, quantitative interpretation of the size differences of certain absorption bands is only possible with very well-matched samples.
Infrared analysis does, however, provide valuable information from small samples about the general nature of a compound of completely unknown composition and origin, with a minimum of preparation. Hence, even though much of the following interpretation of the analysis is speculative, the treatment plan is not affected by these uncertainties. The conservator did not ask for a positive identification of the unknown compound as it was not required, while the definitive results of the analysis were a sufficient basis for treatment decisions.
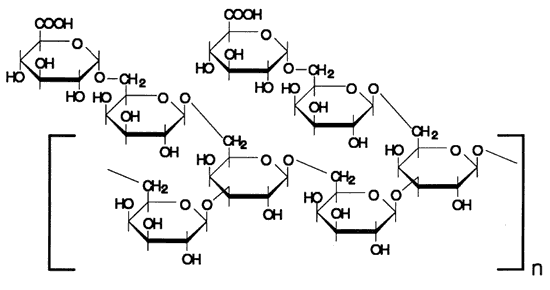
Figure 4. Example of structure possibly occurring in arabic acid, following Aspinal (1970) and Smith and Montgomery (1956)
The overall features of the infrared spectrum of the unknown granular material leave little doubt that it is a polysaccharide. The match of the unknown with authentic gum arabic is very good. The prominent band at 1740 cm-1 is usually identified with absorption due to carbon oxygen double bond (c=O) vibrations (Liang et. al. 1960, Fengel and Ludwig 1991). In vegetable gums, this band can be attributed to the COOH-groups of the many glucuronic acid monomers present in the molecule. A simplified schematic of the structures typical in vegetable gums is shown in (Figure 4. This type of compound occurs with many natural variations. They have in common a high degree of branching and the presence of a significant number of uronic acid monomers. Many compositional and structural variations will have only small effects on infrared spectra.
The evidence presented above leads to the conclusion that the granular material adhering to the paper is a polysaccharide that was added to the papermaker's vat. Used this way, such compounds serve both to prevent entanglement or clumping of fibers in the vat and to bind fibers in the finished sheet (Hughes 1978). According to Hughes (1978), the Japanese name of the compounds used in this way is nori, which is a collective name for "sticky goops," including proteinaceous glues. During discussions at the AIC meeting in Albuquerque, conservators mentioned that starch was the additive used in Chinese papermaking. It was not clear whether this, too, is meant as a collective name for polysaccharide based binders or actually refers to alpha-D-glucan, i.e. actual starch. This uncertainty prompted a search for infrared spectra of starch in the literature for a comparison.
A published spectrum (Pouchert 1975) does indeed resemble slightly those presented in (Figure 3, as well as a galactan (ex gum arable) spectrum found in the same reference and prepared by the same method (Nujol mull). This is somewhat surprising as it is not clear how pure, unoxidized alpha-D-glucan could have such a strong c=0 stretch absorption. Nevertheless, it is possible that the granular material is indeed starch. It is, however, clear that the unknown substance is not a proteinaceous material, e.g. gelatin.
The granular material was wetted during the life of the paper, as evidenced by the tide stains. It seems to have been severely dried at some later time, as indicated by its present behavior towards water and some features of the infrared spectrum. Where freshly prepared gum spectra show only shoulders, the old material shows distinct peaks. This appearance is especially pronounced in the regions from 3600 cm-1 to 3200 cm-1, and 1300 cm-l to 1000 cm-1. Such sharpening of peaks may be explained by the absence of hydrogen bonds to water, which would tend to broaden the resonance. When Keiko Keyes learned about these observations, she inquired to the curator in charge of the documents about possible causes of dehydration. She found out that the statue apparently had been in a fire, according to a conservator at the New Orleans museum of Art who had been treating the wood of the statue (Keyes 1989 B).
Severe drying of a branched polysaccharide will lead to entanglement of the branches. The many hydroxyl groups in the material will hydrogen-bond with each other. The material will form spherical particles due to surface tension, thereby minimizing the surface area per unit mass. The combination of these effects can change the behavior of such polysaccharides towards water in the way observed here for the unknown material. Chemical reactions like ester condensation or oxidative coupling may also occur and would similarly lead to the observed behavior.
While the polysaccharide binder of the paper is desiccated and does not admit water any more, the linen fibers contain a significant amount of bound water, as shown by the infrared spectra (see above). If the fibers were desiccated during their history, they must have been able to reabsorb water, which would mean that physical or chemical changes in the fibers were not as severe as in the granular material. Measurement of the sorption curves of the papers would reveal the extent of such changes. If, on the other hand, the adsorbed water detected in the fibers is trapped in inaccessible internal cavities, it may never have been removed from the fiber, even in severely desiccating conditions.
One can see from (Figure 1 that the fibers in these papers, as in many historic papers, show no signs of mechanical damage. This would make it possible for internal microcavities, holding trapped water, to exist. It may also exclude pollutants like SO2 and other potentially harmful chemicals from most of the cellulose. In the paper investigated here, interfiber bonding is achieved by adding polysaccharide binder. In modern, machine made papers, interfiber bonding is achieved by beating fibers to open up the structure and fibrillate the cellulose (see, e.g., Centola 1970). Cellulose molecules in beaten fibers are more accessible and mobile, which is necessary for acid hydrolysis to proceed (see Harris 1975, or, for a review, Berndt 1987). One can assume that the absence of mechanical damage to the linen fibers of these papers contributed significantly to their durability.
Since the granular material is apparently a remnant of the papermaking process and an intrinsic part of the paper, it is not expected to pose any danger to the documents. Moreover, it is part of the history of the documents. Accordingly, the treatment plan specified that it not be removed from the documents. The solubility test indicated that the material would not react to water, but it may be soluble in alkali. It was therefore decided to refrain from alkaline washing and/or deacidification treatments. The treatment plan specified keeping moisture application to a minimum, though, to avoid altering the appearance of the documents, especially of the paper tinted yellow.
Brief Summary of Treatment
The documents were mechanically cleaned and loose dirt removed by vacuum. They were carefully washed using a humidifier on a vacuum table. Broken folds were mended and some edges were strengthened. Much of the brittleness of the paper disappeared on cleaning because encrusted dirt was removed (Ogden 1991). It should be assumed that some of the "dirt" was actually desiccated binder (the granular material). As much as possible of the removed material was saved and sent to the New Orleans Museum of Art (Ogden 1991), and may therefore be available for analysis to an interested party. The document papers were significantly brightened by the treatments.
Considering that these 800 year old papers had literally gone through fire and water, and were exposed to nearly all detrimental influences except light, it is remarkable how well they survived. As mentioned above, their durability may be partly attributed to a manufacturing process that avoids mechanically damaging the fibers.
References
Aspinall, G. O. 1970. Pectins, plant gums, and other plant polysaccharides. In The carbohydrates, ed. W. Pigman et. al. New York: Academic Press. 515-536.
Berndt, H. 1987. The role of wood in interactive interfaces: the chemistry. Journal of Wood Conservation 2(1):21-41
Burke, J. W. 1989. Personal communication.
Centola, G. 1970. Beating and refining -- action upon fibers. In Pulp and Paper Technology, ed. K. W. Britt. New York: Van Nostrand Reinhold Company.
Erhard, D., W. Hopwood, M. Baker and D. von Endt. 1988. A systematic approach to the instrumental analysis of natural finishes and binding media. AIC preprints, 16th Annual Meeting, American Institute for Conservation, New Orleans, LA. 67-84.
Fengel, D. and M. Ludwig. 1991. Möglichkeiten und Grenzen der FTIR-Spektroskopie bei der Charakterisierung von Cellulose. Das Papier 45(2): 45-51.
Harris, J. F. 1975. Acid hydrolysis and dehydration reactions for utilizing plant carbohydrates. Applied Polymer Symposium 28:131-144.
Hughes, S. 1978. Washi: The world of Japanese paper. San Francisco: Kodansha International. 84.
Keyes, K. M. 1989 A. Report and estimate, submitted to the New Orleans Museum of Art, 16 May, 1989.
Keyes, K. M. 1989 B. Personal communication.
Ogden, L. K. 1990. Personal communication.
Ogden, L. K. 1991. Personal communication.
Pouchert. C. J. 1975. Aldrich library of infrared spectra, 2nd edition. Aldrich Chemical Co. Inc.
Smith, F. and R. Montgomery. 1959. The chemistry of plant gums and mucilages. New York: Reinhold Publishing Corporation.
Stamm, A. J. 1964. Wood and cellulose science. New York: The Ronald Press Company. 177
Wood, D. K. 1986. 75th Anniversary Gift: A Twelfth-Century Chinese Bodhissattva. Arts Quarterly VIII(4): 19-21. New Orleans: Museum of Art.
Harald BerndtAssistant Specialist
Timber Mechanics/Wood Artifacts Conservation Group
University of California Forest Products Laboratory
Publication History
Received: Fall 1991
Paper delivered at the Book and Paper specialty group session, AIC 19th Annual Meeting, June 3-8, 1991, Albuquerque, New Mexico.
Papers for the specialty group session are selected by committee, based on abstracts and there has been no further peer review. Papers are received by the compiler in the Fall following the meeting and the author is welcome to make revisions, minor or major.