Relationships Between Colour Production in Cellulose and the Chemical Changes Brought About by Bleaching
By Helen D. BurgessTranscript of a Lecture given at the Meeting of the Book and Paper Specialty Group, AIC Annual Meeting, Milwaukee, May 27 - 30, 1982
I. Introduction
This lecture discusses how the chemical changes brought about by bleaching influences yellowing or colour production in paper fibres. This is an important topic for conservators to understand, not only because of aesthetic considerations, but also because these phenomena are intimately related to deterioration and loss of permanence of the paper support.
The main application of this topic is going to be to the standard methods with which all of you are familiar. These include:
hypochlorite at various pH values
chloramine-T
chlorine dioxide - both immersion and gaseous stabilized
hydrogen peroxide
However, much of the information has equal application to photochemical methods of bleaching and I shall be drawing some correlations as I go along.
II. Colour Production During Bleaching
The first type of yellowing to be discussed is an immediate production of colour during the actual bleaching process itself. This is a rather narrow category which really only applies to the use of chlorine type bleaches with ligneous wood pulp paper. The cause is the formation of stable chlorinated fibres which are yellow in colour.
There are three examples which fall into this group. The first is acidic hypochlorite. In this case, yellowing is a particular problem when very short bleaching times must be used. A second example is chlorine dioxide. Most commonly, colour production, which can range from a definite pink to yellow brown, is seen with the gaseous method although it may also be observed with immersion methods as well. For immersion methods, colour production can occur if the bath is used after it begins to be exhausted. Experimentally, I have found that the brightening is very fast when the bath is fresh, but that it decays rapidly until a point around 0.5 hr when actual yellowing of the ligneous paper may result. A third example where yellowing has been observed as a direct and immediate result of the treatment is chloramine-T. This is probably a concentration effect or perhaps is due to a particularly stable chlorine intermediate.
III. Colour Production After Bleaching
The second type of yellowing I would like to discuss is that which occurs during storage of experimental samples or artifacts. It may be accelerated by exposure to light, but more specifically it is the result of degradation and chlorination of the paper fibres during the bleaching process. Of course, the extent varies with the type of paper under investigation as well as the particular bleaching conditions of the treatment selected.
In reference to degradation, the yellowing is directly related to the oxidation occurring during bleaching. In particular, the production of carbonyl functional groups such as aldehyde, ketone and carboxylic acid have been credited with leading to coloured fibres. It must also be noted that these same oxidation products have been shown to also be caused by photochemical exposure.
The second cause of yellowing, that is, chlorine residues, can only apply when a chlorine-containing bleach is used. In order of importance, in terms of extent of chlorination possible, there is the acidic pH 4.5 hypochlorite bleach, followed by neutral hypochlorite and finally an alkaline pH 9 bath. Considerably less chlorine remains in the fibres with the chlorine dioxide methods.
I would like to go into this subject in a little more depth now, and relate it specifically to the actual structures found in paper. I shall consider mainly the lignin and cellulose fractions here, but most of you are aware of the fact that paper may also contain a certain amount of natural wood extractives and hemi-cellulose as well. However, these latter components tend to be removed in all but the least extensive of the pulping processes. Any residues will participate in reactions similar to that observed for lignin and cellulose.
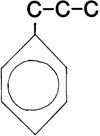
The lignin fraction is made up of polymeric molecules which are composed of phenylpropane building blocks (see below). The individual units can be strung together in various ways and the phenyl group will usually contain hydroxyl -OH and methoxy -OCH3 substituents.
Figure 1: Structure of lignin
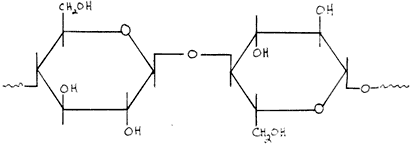
Cellulose, on the other hand, is a more uniform polymer made up of glucose units attached through a B-1-4 linkage (shown below).
Figure 2: Structure of cellulose
Both compounds are subject to oxidation and chlorination with a resulting general degradation and colour formation. However, the lignin is much less stable than the cellulose and makes a much greater contribution to fibre deterioration.
This generalization may be extended to include their individual response to the degradative effects of sunlight. Lignin is readily transformed at the wavelengths found in natural light, but very pure cellulose absorbs only weakly. However, even slight oxidation will increase its sensitivity to photo-degradation. The presence of lignin, metal salts and other impurities will have a similar effect. It should be noted that these comments also apply to possible degradation from conventional oxidizing agent bleaches like hypochlorite, peroxide and so on.
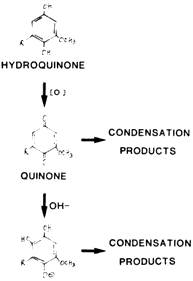
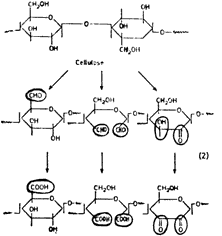
The next two figures show some of the important chemical transformations responsible for degradation and colour formation. Figure 3 illustrates a lignin monomer in its unoxidized hydroquinone form. It is shown being oxidized to the quinone grouping which reacts further to form coloured condensation products. The application of alkali will have a similar result. In Figure 4, the hydroxyl -OH groups on the glucose monomers are shown as they are being oxidized to various carbonyl groups such as the aldehyde -CHO, ketone c=O, and acid groups, -COOH.
Figure 3:
Figure 4:
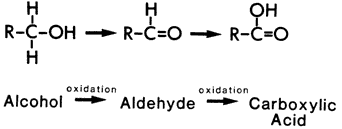
Two important causes of this oxidation are air oxidation and chemical bleaches. The air oxidation increases in importance when the fibres have become destabilized by some initial degradation. The oxidative reactions tend to be progressive, with identifiable intermediates. For example, the hydroxyl group in cellulose will progress from its initial alcohol oxidation state to first an aldehyde and then a carboxylic acid.
Figure 5: Progressive oxidation of cellulose
All of this information concerning the chemistry of oxidation is meaningful only when it can be related to the extent of degradation and ultimately to some sort of sensible approach to conservation treatments. Therefore, I wish to briefly discuss how the various bleaches compare in terms of oxidation, so that we can gain a better appreciation of the relative risks of different treatments.
The relative order of five different bleaches in terms of increasing oxidation of the cellulose fibre is:
stabilized hydrogen peroxide
chlorine dioxide
pH 9.0 hypochlorite
pH 4.5 hypochlorite
pH 7.0 hypochlorite
The rate of oxidation may be immediately correlated to rate of fibre degradation which, in turn, is reflected in the rate of yellowing or colour reversion.
The direct relationship between the extent of colour reversion and rate of degradation is made clear by comparing the relative orders of increasing rates of colour reversion with increasing fibre degradation for linen rag fibres (Table I).
| Increasing Reversion | Increasing Fibre Degradation |
|---|---|
| stabilized hydrogen peroxide | stabilized hydrogen peroxide |
| chlorine dioxide | chlorine dioxide |
| pH 9.0 hypochlorite | pH 9.0 hypochlorite |
| pH 4.5 hypochlorite | pH 4.5 hypochlorite |
| pH 7.0 hypochlorite | pH 7.0 hypochlorite |
The order is the same in both cases.
When one is considering pure linen fibres such as in this situation, chlorination is not as important as a cause of yellowing. Instead, everything we have been discussing can be related to a quantity known as the oxidation potential of the system. The higher the oxidation potential, the more likely it is that oxidation will take place. The potential is affected by such things as concentration, temperature and pH. In particular, the acidity may affect oxidation since it may influence the chemical reactions which make up the oxidation reactions.
A good example of this is the chemical system based on hypochlorite bleach. The oxidation potential varies incredibly with pH, with the maximum potential coming around neutrality (pH 7). With this information at our disposal, it is predictable that the rate or extent of degradation of cellulosic fibres bleached by hypochlorite at various pH values is going to closely follow the oxidation potential data. The literature shows this to be correct with maximum degradation and oxidation to be at around pH 7. Since oxidation leads to yellowing or colour reversion, the observation that most rapid reversion is also at pH 7 is not surprising.
The next question which one may ask is whether the reactions at the various pH levels differ only in their rate or are there actually different chemical mechanisms at work, with different intermediates and different end-products. The answer is that in this case at least, pH has a profound effect not only on the rate of oxidation, but also on the type of oxidation taking place.
Two of the most important functional groups produced by oxidation are the aldehyde carbonyl and the carboxylic acid species. It has been verified experimentally that their relative quantities do vary with pH: the aldehyde is formed preferentially at low pH while the carboxylate is formed at alkaline pH values. It is clear that the chemistry of oxidation at various pH's must be very different.
These differences are especially important when one considers the rate of yellowing of papers which contain various types of oxidation products. The general scientific opinion right now is that they all contribute to yellowing, but perhaps not to the same extent. For example, carboxylate groups have generally been shown to cause less reversion than the carbonyl aldehyde and ketone groups. If these aldehyde and keto groups are reduced back to the hydroxyl, as in treatment with borohydrides, yellowing will be greatly diminished. It has been experimentally shown that yellowing will increase as the carbonyl content of the fibres goes up. It will, however, be lower at all carbonyl concentrations when the fibres have been subjected to a borohydride reduction.
Relating colour production in fibres to oxidation really works best when pure cellulosics such as linen or cotton are being discussed. When the paper is a wood pulp type, the lignin component makes chlorination of the fibres very important. Chlorination refers to a process in which a chlorine-containing bleach will react with a fibre to form a chlorine-containing complex which can then break down to yield an oxidized fibre. However, not all of these chlorine complexes break down during bleaching. Instead, they remain part of the fibre and lead to greatly increased colour reversion.
The rate of chlorination can also be affected by pH. For example, the most effective chlorinating hypochlorite is the acid system, followed by the neutral bleach and then the alkaline hypochlorite. In terms of its chemistry, the chlorine, C12 form predominates at low pH, the hypochlorous HOC1 at neutral pH and the hypochlorite OC1- at
alkaline values. The chemical reagent changes with pH and hence, so does the mechanism of reaction as well as the rate of chlorination. The following table summarizes how the type of bleach used on a mixed fibre wood pulp and linen paper may influence chlorination:
| Increasing Reversion | Increasing Fibre Chlorination |
| stabilized hydrogen peroxide | stabilized hydrogen peroxide |
| chlorine dioxide | chlorine dioxide |
| pH 9.0 hypochlorite | pH 9.0 hypochlorite |
| pH 7.0 hypochlorite | pH 7.0 hypochlorite |
| pH 4.5 hypochlorite | pH 4.5 hypochlorite |
In conclusion, I would just like to emphasize that the rate of colour production in paper is going to depend enormously on the chemical changes which the conservator introduces into the fibres. The initial artifact condition and fibre content is going to increase these differences rather than diminish them. However, by gaining an understanding of what the various bleaching systems do to the fibre and, as importantly, how the detrimental effects may be minimized, will go a long way to helping the conservator make the necessary decisions.
Bibliography
Abadie-Maumert, F.A. and Loras, V., "Comparison of Accelerated Test Methods For Determining Brightness Stability and Long-Term Aging of Bleached Sulphite and Sulphate Pulps", Norsk Skoginduotri, Vol. 23, No. 4, 1979, pp 75-79.
Birtwell et al., J. Textile Instit., 1925, p. Til.
Burgess, H.D., "The Colour Reversion of Paper After Bleaching", preprints for Cambridge 1980: The Conservation of Library and Archive Materials and the Graphic Arts, 1980, pp. 111-183.
Burgess, H.D. & Hanlan, J.F., "The Degradation of Cellulose in Conservation Bleaching Treatments", J.IIC-Canadian Group, Vol. 4, No. 2, 1979, pp. 15-22.
Corbi and Rapson, Pulp and Paper Mag. of Canada, 1964, p. T467.
Feller, R.L., "Notes on the Chemistry of Bleaching", Bulletin of the American-Group - IIC, No. 2, 1971, pp. 39-56.
Hoist, Gustaf, "The Chemistry of Bleaching and Oxidizing Agents", Chemical Reviews, Vol. 54, 1954, pp. 168-194.
Hosokawa, Jun, et al., "Studies on Colour Reversion of Ozone-Bleached K.P. II. Functional Groups of Ozone-Bleached K.P.", J. Japan Wood Research Soc., Vol. 24, No. 9, 1978, pp. 638-642.
Howard, E.J. & Histed, J.A., "Pulp Brightness Reversion", Tappi, Vol. 47, no. 11, Nov. 1964, pp. 653-662.
Lewin, M. and Epstein, J.A., "Functional Groups and Degradation of Cotton Oxidized by Hypochlorite", J. Poly. Sci., Vol. 58, 1962, pp. 1023-1037.
Lin, S.Y. and Kringstad, K.P., "Stabilization of Lignin and Lignin Model Compounds to Photodegradation", Tappi, Vol. 53, No. 9, 1970, pp. 1675-1677.
Varshney, M.C. and Luner, P., "Reactions of Sodium Borohydride as Applied to Pulp and Paper", Tappi, Vol. 44, No. 4, 1961, pp. 285-289.
Helen D. BurgessConservation Processes Research
Canadian Conservation Institute
Publication History
Received: Fall 1982
Paper delivered at the Book and Paper specialty group session, AIC 10th Annual Meeting, May 26-30. 1982, Mailwaukee, Wisconsin.
Papers for the specialty group session are selected by committee, based on abstracts and there has been no further peer review. Papers are received by the compiler in the Fall following the meeting and the author is welcome to make revisions, minor or major.