Methylcellulose & Sodium Carboxymethylcellulose: Uses in Paper Conservation
by Cathleen BakerThis paper concerns the many uses of these two polymers in conservation. Although this information is paper conservation oriented, it may very well be that it can be useful to conservators in other fields.
Methylcellulose (MC) and sodium carboxymethylcellulose (sod. CMC) have many other uses besides those in conservation. A brief rummage through your medicine cabinet may come up with such products as toothpaste, laxatives, or diet pills each of which may contain either MC or sod. CMC. Other products include ice cream, water-based paints, detergents and a variety of paper products to name but a few. Characteristics which make them useful are: high viscosity in low concentrations, defoaming abilities, surfactant, and bulking abilities. They are not toxic and do not promote allergic reactions in humans. At the end of this paper, I have given more technical information on the composition of the various MCs and sod. CMCs.
These cellulose polymers can be purchased in grades ranging from coarse to fine particles and in varying viscosities. In solution, Hercules CMC 7H and Culminal (MC from Talas) are quite clear while Cellofas B3500 from Conservation Materials is hazy.
The easiest way to make up any of the MCs or sod. CMCs is to measure out the desired quantity of the powder, fill a blender with the right amount of deionized or distilled water, turn on the blender to the lowest speed, and pour the powder in a steady stream into the vortex. As soon as all of the powder is in the water, turn off the blender. Over-blending can result in a loss of viscosity. No preservative is necessary as long as purified water is used and the storage container is airtight. After blending, it is best to leave the solution at least one hour before using.
Most conservators regard the MCs and sod. CMCs primarily as adhesives. We will see in fact that they have many other uses, but let's start with their adhesive applications. First may I say that neither MC or sod. CMC alone is really strong enough to be used as an adhesive when a great deal of stress on the bond is encountered such as in tear repairs or hinges.
MC should also not be used for an overall backing as it is not a very polar adhesive and as such will not affect a very good bond between papers, especially smooth-surfaced papers. It is occasionally mixed with wheat starch paste in order to provide 'slip' and indeed this mixture comprises most wallpaper pastes. Sod. CMC on the other hand is a very polar adhesive and as such makes a very good bond between sheets of paper and is useful for overall backings where stress on the bond is not a real problem. It could also be mixed with wheat starch paste to provide 'slip'. The advantage sod. CMC has over wheat starch paste is that a dry backing can be done when the original work of art consists of water sensitive media or paper which is dimensionally unstable. This is because a 2.5% solution of say CMC 7H is very viscous but is not very 'wet' so that it can be brushed on the reverse of the original and the backing paper without either expanding too much or without much water penetrating to the surface of the original. Once the backing is complete, air drying can take place with the original face down so that water evaporates from the back reducing still further the risk of the front getting wet. When dry the backed original can be humidified and pressed or put out on a drying screen/board to flatten. The adhesive dries to a very thin even layer and is easily reversible with cold water. It is non-staining and does not become brittle upon ageing. Other applications in this section might include temporary facings, mends or backings. If you can use hot water for a wash bath, you might like to use MC as the temporary adhesive as it will not dissolve in hot water but is easily reversed in cold water. This would probably hold together a badly torn piece which could not be handled in any other way.
An extremely useful method for quickly filling small holes or losses in paper is accomplished by mixing Whatman Cellulose Powder CF11 with MC or sod. CMC. The cellulose powder as sold is very white, but gradations of browns can be made by cooking the dry powder in a Teflon pan over a hot plate. Wear a dust mask as the powder can be irritating if inhaled. Also be careful not to scorch the powder. The cooked powder can then be color matched, dry, to the original paper by adding lighter or darker shades of the powder as necessary. Then enough 3% MC or 2.5% sod. CMC is added to make a stiff paste. It can then be applied to the hole or loss with a microspatula, tip of a scalpel blade, etc. Leave it to air dry. Retouching is seldom necessary if you have matched the powder color well in the first place. If a smoother surface is needed, small amounts of calcium carbonate can be added to the powder before the adhesive is added. Any excess of the cellulose powder paste left over can be allowed to dry out, and later on, rejuvenated for further use by adding a few drops of water and working up into a paste again.
These cellulose polymers also act as deflocculating agents in that they cause particles such as fibers to stay in suspension and not clump together and separate out of solution. This advantage can be very useful in employing wet pulp fills in a treatment, especially when using a pipette to distribute the pulp from the slurry. The proportions are about 1:3, .5% MC or sod. CMC: pulp slurry.
In low concentrations such as .5%, these polymers can serve very well as sizing agents and indeed are used extensively in the paper industry as both internal and surface sizes. In paper conservation, the use of a sizing agent can perform two roles: to repel oil and/or grease and to enhance the fiber-to-fiber bonding which makes the paper stronger and more durable. The .5% solutions of either MC or sod. CMC can be brushed on both sides of the non-water sensitive original through tissue paper on silicone paper and left to air dry. They could also be brushed on the surfaces while the piece was on the vacuum suction table to enhance penetration. This should be done on a porous yet slick-surface material such as Hollytex, Reemay or Pellon and allowed to air dry.
Because of its surfactant properties, MC and sod. CMC can be used like detergents. If a swab is dipped in the viscous solution and the excess wiped off, it can be used as a kind of cleaning lubricant over areas of dirt and/or staining with little or no risk of abrading the paper. Excess on the paper surface should be removed when the treatment is complete as both MC and sod. CMC will leave a slightly greyish film.
In conjunction with this surfactant property and because 3% or 2.5% solutions carry a lot of water but are not wet, these polymers can be used as poultice material to soak up staining, soften adhesives through paper (even varnished paper), aid in removing old adhesive residues without affecting water sensitive media, and can act as a viscous carrier for enzymes, bleach and solvents.
These are some of the most common applications which one might use in paper conservation. There are probably many more uses for these very versatile products and I encourage you to try them.
The following is some technical information which you may find useful.
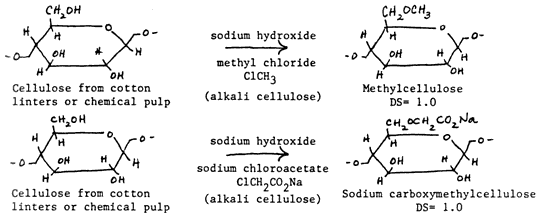
Both methylcellulose and sodium carboxymethylcellulose are cellulose ethers made from alkali cellulose which has been chemically altered so that a substitution of either -CH3 or -CH2000Na is made for the proton (H) in the -OH hydroxyl groups on the glucose unit of the cellulose polymer. EXAMPLES:
The solubility of these ethers in water or organic solvents is dependent upon the degree of substitution or DS of the -CH3 or -CH 2COONa for the number of protons in the hydroxyl groups. The maximum DS would be 3.0 but this is not possible to achieve. Average DS values are between 1.3-2.6 for MC and 0.38-1.40 for sodium CMC. Generally the lower levels of DS are soluble in aqueous or alkali solutions; the intermediate ranges are soluble in cold water; and the higher DS values indicate increasing solubility in organic solvents.
As used in paper conservation, MC is soluble in cold water but not in hot water (either as the dry powder or as the dry film). Sod. CMC is soluble in either cold or hot water. Both, in an aqueous solution, can be diluted to some degree by organic solvents such as ethanol or acetone. Too much solvent however will result in the polymer precipitating out of solution.
Common MCs used in paper conservation are Culminal (Henkel & Cie, G.m.b.H., Germany); Methofas (Imperial Chemical Industries, England); and Methocel (Dow Chemical Co., USA). Talas supplies Culminal. Light Impressions and Fisher Scientific supply an unknown type. I do not have any more technical information about the above MCs.
Common sod. CMCs used in paper conservation are CMC 7H Cellulose Gum (Hercules, Inc., USA) and Cellofas B3500 (I.C.I., England). The latter is no longer manufactured but is still available from Conservation Materials, Ltd. CMC 7H is available from the manufacturer (ask for a sample) and from Fisher Scientific (CMC 7HSP). The following is some technical information on the above. CMC 7H has a DS range of 0.65-0.85 with a sodium content of 7.0-8.5%. It has a high viscosity at 1% concentration of 1500-2500 centiposes at 25°C. The refractive index of a typical air dried film is 1.515 (glass has a R.I. of 1.5-1.7; water at 25°C is 1.332). The pH of a 2% solution is 7.5. Cellofas B3500 is is 97.54% sodium carboxymethylcellulose, 0.98% sodium chloride and 1.48% sodium glycolate (information from a letter from Douglas Adams, Conservation Materials, Ltd., Dec. 7, 1978). Cellofas B3500 has been used in England as an adhesive and sizing agent for well over 12 years and has no known adverse effects.
It is not intended by this paper to suggest that either MC or sod. CMC be substituted for well established adhesives such as wheat starch paste. Rather the information is presented to give paper, book and other conservators a wider choice of materials to tackle the seemingly endless problems we encounter daily. There has been some question as to the suitability of cellulose ethers, especially sod. CMC, in paper conservation and it is apparent that more research must be done into all adhesives used in conjunction with paper. Dr Robert Feller has done research into the films of adhesives in which the cellulose ethers such as MC and sod. CMC came out as stable materials. However, testing materials in the real situation of conservation treatment, backings and sizing of papers, has yet to be done in a systematic and scientific way. To start the ball rolling, I would appreciate any information from conservators who have used these polymers and their findings and/or feelings concerning their use. Perhaps by the next meeting of the Book and Paper Group, I will have something further to report.
Bibliography
1. Batdorf, J.B. and J.M. Rossman. "Sodium Carboxymethylcellulose," Roy L. Whistler, ed. Industrial Gums. Academic Press: NY, 1973, p.695-729.
2. Browning, B.L. Analysis of Paper. 2nd ed. Marcel Dekker: NY & Basel, 1977. p. 273-75.
3. Feller, R.L. "Evaluation of the Stability of Cellulosics." Talk given at the AIC Conference, Philadelphia, PA, May 1981.
4. Greminger, G.K. and A.B. Savage. "Methylcellulose and Its Derivatives." Whistler, Industrial Gums. p. 619-648.
5. Hercules, Inc. Technical information booklets.
6. Ott, E., H.M. Spurlin and M.W. Graffin. Cellulose and Cellulose Derivatives. Interscience Publ.: NY, 2nd ed. 1954. Part II, p. 937-45. 7. Rudolf, A.V.R., I.W. Herrick and M.F. Adams. "Archives Document Preservation". Northwest Science (1966), 40, no. 1. 8. Stecher, P.G. ed. The Merck Index. 8th ed. Merck & Co.: Rathway, 1968. 9. Stuhrke, R.A. "The Development of Permanent Paper." John Williams, ed. Preservation of Paper and Textiles of Historic and Artistic Value. American Chemical Society: Washington, DC, 1977. p. 35.
Cathleen BakerAssistant Professor
Art Conservation Program
Publication History
Received: Fall 1982
Paper delivered at the Book and Paper specialty group session, AIC 10th Annual Meeting, May 26-30. 1982, Mailwaukee, Wisconsin.
Papers for the specialty group session are selected by committee, based on abstracts and there has been no further peer review. Papers are received by the compiler in the Fall following the meeting and the author is welcome to make revisions, minor or major.