Enzymes
Foundation for Advancement in Conservation

© 2008
Introduction
Enzymes are often used in paper conservation to aid in the removal of adhesive residues from previous repairs or to facilitate the removal of poor-quality secondary backing supports and mats.
Art conservators need to understand the chemistry of enzymes to select and use them appropriately.
Understanding enzyme chemistry requires a basic knowledge of organic chemistry, chemical bonding, functional groups, and acids and bases. Organic Chemistry I for Dummies by Arthur Winter is a useful information source.
Contents
This tutorial is divided into the following sections:
Please complete each section in order, as the information builds on that covered in previous sections. You can return to any section later.
Press Ctrl+Shift+F to search.
Learning Objectives
After completing this tutorial, you will be able to:
- Describe the chemical composition of enzymes
- Understand the difference between the primary, secondary, tertiary, and quaternary structures
- Characterize the functions of enzymes, co-enzymes, and co-factors
- State the functions of three significant types of enzymes in art conservation: amylase, lipase, and protease
Chemical Composition of Enzymes
In this section you will learn that:
- Enzymes are proteins.
- Proteins are macromolecules composed of linear chains of amino acids joined by peptide bonds.
- Twenty amino acids are found in nature.
- Amino acids may be non-polar, neutral polar, or charged polar.
Chemical Composition of Enzymes: Proteins
- Enzymes are proteins. They are unbranched, linear molecules made from amino acids.
- Proteins are huge macro-molecules with a molecular mass between 10,000 and 1,000,000. They can also be referred to as polypeptides.
- A molecule made of amino acids with a mass of less than 10,000 is called a peptide.
Chemical Composition of Enzymes: Amino Acids
With one exception all amino acids have this form.

Amino Acids: Proline
The exception is proline where the side chain C3H6 folds back to join the nitrogen atom and form a ring.

Amino Acids: Zwitterions
There are 20 amino acids. In water they exist in a dipolar, or zwitterionic state.

Amino acids differ by their side chains, and it is the side chains, R, that determine the polarity of the amino acid.
- Non-polar
- Neutral polar
- Charged polar
Amino Acids: Non-Polar
Non-polar: The side chain atoms have no lone pairs for hydrogen bonding.

Amino Acids: Neutral Polar
Neutral polar: These amino acids have lone pairs for hydrogen bonding.

Amino Acids: Charged Polar
Charged polar: These amino acids have a permanent partial charge.

Chemical Composition of Enzymes: Peptide Bonds
The amino acids join together via peptide bonds.

Chemical Composition of Enzymes: Peptide Structure
A protein composed of amino acids joined with peptide bonds has the following chemical composition:

Chemical Composition of Enzymes: Summary
In this section you learned that:
- Enzymes are proteins.
- Proteins are macromolecules composed of linear chains of amino acids joined by peptide bonds.
- The twenty amino acids found in nature differ only in the side chain attached to the alpha carbon.
- Amino acids may be non-polar, neutral polar, or charged polar.
Protein Structure
In this section you will learn that:
- Proteins have primary, secondary, tertiary, and quaternary three-dimensional structures.
- The three-dimensional structure is held together by hydrogen bonding between side chains.
- Proteins that contain cysteine may also include crosslinks formed between the cysteine units.
- The three-dimensional structures allow proteins to form active sites that have a particular geometry for substrate molecules to fit like a key in a lock.
Protein Structure: Primary Structure

A simple list of the order in which the different enzymes are joined in a protein is called the primary structure.
This is the primary structure of a chicken protein.
The cysteine amino acids (Cys) can crosslink (covalent bonding) if they are close.
Protein Structure: Cysteine Crosslink

The cysteine amino acid contains sulfur at the end of its side chain. This will form a covalent bond with the sulfur on another cysteine molecule.
These sulfur bonds are attractive (tasty) to clothes moths.
They are found in wool but not in silk.
Protein Structure: Secondary Structure
The polypeptide chain will fold either into:
- A helix with side chains on the outside
- A sheet structure
Both of these structures are held together by hydrogen bonding and are called the secondary structure.
Protein Structure: Tertiary and Quaternary Structures
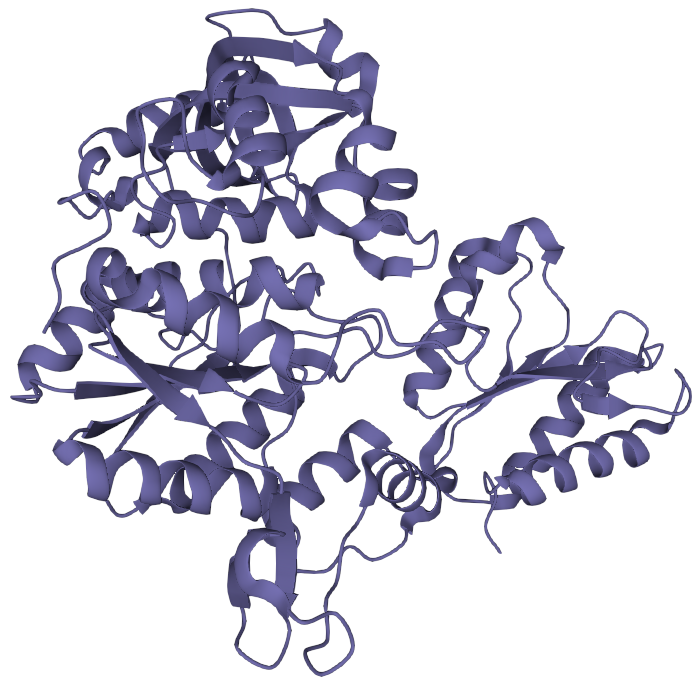
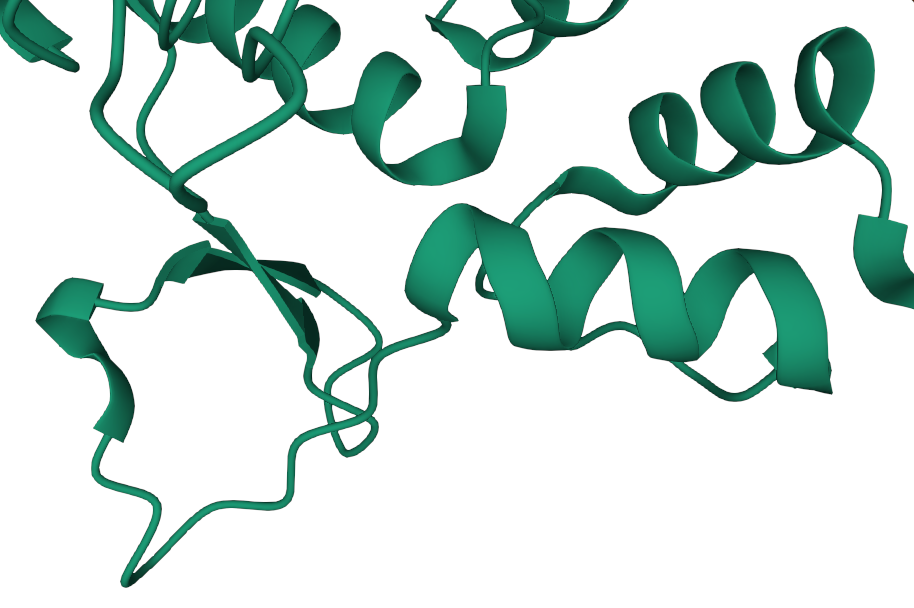
The secondary structure will fold into a three dimensional shape, largely determined by the polarity of the side groups. This is the tertiary structure.
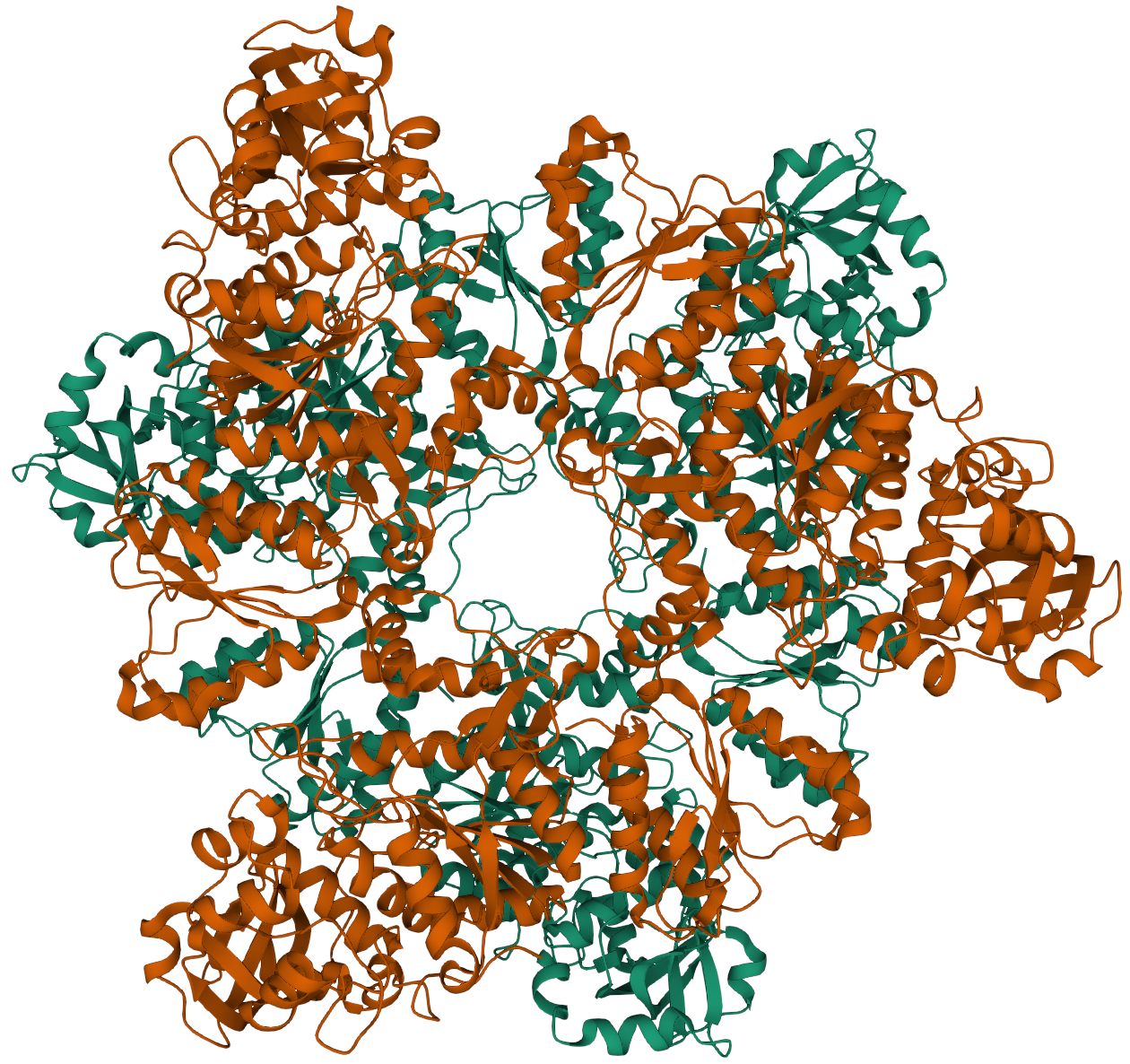
If an enzyme consists of two or more separate polypeptides folded together this is termed quaternary structure.
Protein Structure: Animated View of a Lipase Structure
Protein Structure: Active Sites
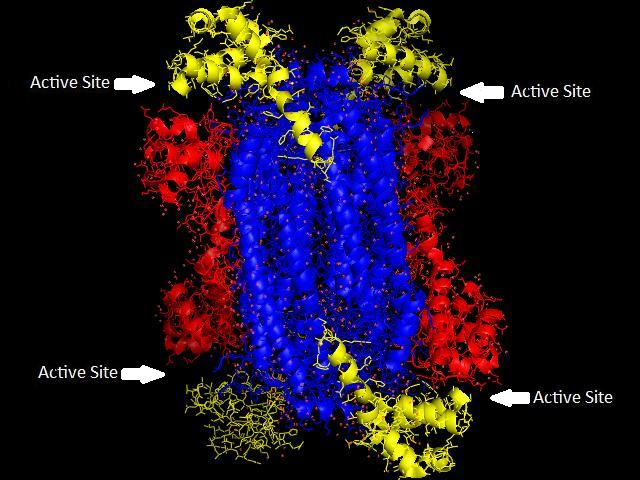
This complicated folding results in a large, multifaceted molecule with active sites.
A single polypeptide chain usually has one active site.
Enzymes with a quaternary structure may have more than one.
Active Sites: Lock and Key
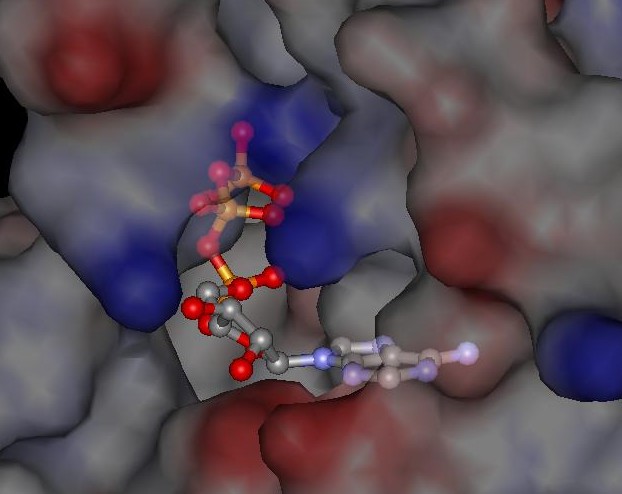
Certain substrate molecules will fit into the active sites, rather like a lock and key.
In fact, since the large enzyme molecule is not rigid, it will bend and conform to accommodate the correct substrate.
Once the substrate molecules are "locked" onto the active sites, the enzyme acts as a catalyst for chemical reactions.
Protein Structure: Summary
In this section you learned that:
- Proteins have primary, secondary, tertiary, and quaternary three-dimensional structures.
- The three-dimensional structure is held together by hydrogen bonding between side chains.
- Proteins that contain cysteine may also include crosslinks formed between the cysteine units.
- The three-dimensional structures allow proteins to form active sites that have a particular geometry for substrate molecules to fit like a key in a lock.
What Enzymes Do
In this section you will learn that:
- Enzymes are biocatalysts.
- Reactants bind to the active sites of the enzyme and are held in the correct conformation for a reaction to occur.
- The catalyst lowers the activation energy for the reaction.
- Sometimes co-enzymes and co-factors are required for enzymes to work effectively.
What Enzymes Do: Definition
An enzyme is a biocatalyst. A catalyst speeds up the rate of a reaction towards completion by lowering the activation energy.

What Enzymes Do: Catalysis
 The enzyme does this by providing a rigid environment in the activation site(s) where the reactant(s) are held in the correct conformation
for the reaction to take place.
The enzyme does this by providing a rigid environment in the activation site(s) where the reactant(s) are held in the correct conformation
for the reaction to take place.
Catalysts do not take part in the reaction. In theory the products are released leaving the enzyme in exactly the same state so that it can be re-used indefinitely.
What Enzymes Do: Catalyst Poisoning
In fact the enzyme is slowly "poisoned" by impurities or the instability of the active site.
This is not a problem in conservation. However, the catalysts used constantly in the cracking of heavy oil last between 6 months to a year.
What Enzymes Do: Activation Energy

In order to react, most reactants undergo a high energy, transitional, conformation state. The active site binds the molecule which reduces rotational and transitional freedom.
If the enzyme-substrate complex resembles the transition state then the free energy difference between the ground state and transition state will be lowered.
What Enzymes Do: Co-enzymes and Co-factors
Co-enzymes:
These are small, organic molecules, often the active forms of vitamins, that are needed for some of the enzymes to work effectively. They are not necessarily bound to the enzyme and can be consumed during the reaction.
Co-factors:
Metal ions that are part of the enzyme and are needed by some enzymes to work effectively.
What Enzymes Do: Summary
In this section you learned that:
- Enzymes are biocatalysts.
- Reactants bind to the active sites of the enzyme and are held in the correct conformation for a reaction to occur.
- The catalyst lowers the activation energy for the reaction.
- Sometimes co-enzymes and co-factors are required for enzymes to work effectively.
Enzymes in Conservation
In this section you will learn that:
- Amylases, proteases, and lipases are the three categories of enzymes that are important in art conservation.
- Amylases break down glucose linkages in starch.
- Proteases break down peptide bonds in proteins.
- Lipases break down the ester bonds in lipids.
- Each of these types of reactions helps create products that are water soluble.
- Enzymes require the proper pH and temperature to work best.
Enzymes in Conservation: Three Important Categories
- Amylase
- Protease
- Lipase
Enzymes in Conservation: Amylase
Amylase helps to break down the 1,4-glucose linkages between repeating units in starch.

α-Amylase is found in animals and β-amylase in plants.
Amylase: Optimal Conditions
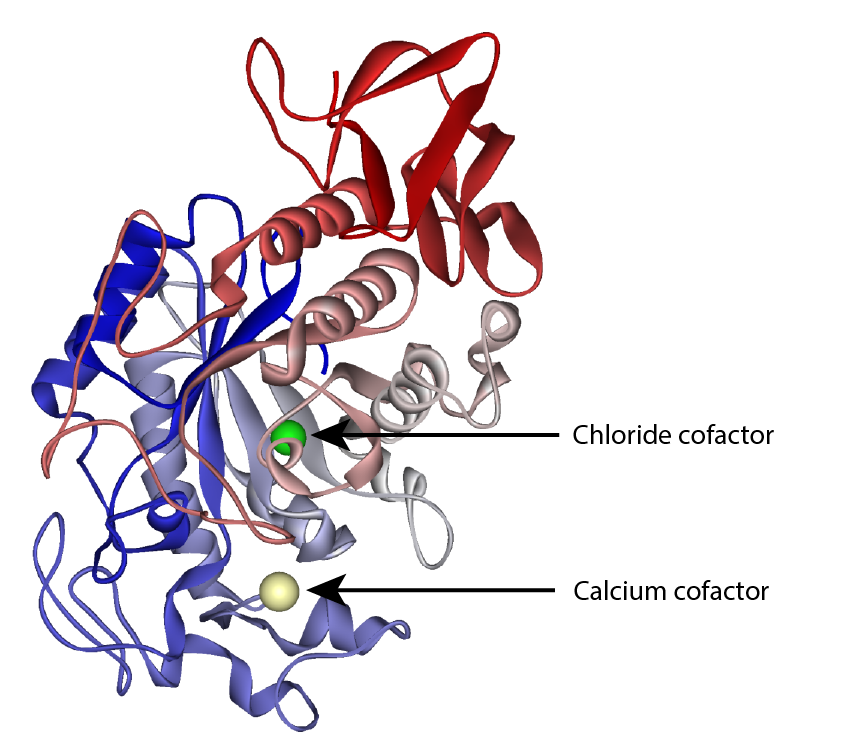 Depending on the source α-amylase works best between pH 5–7 and at around 20 ºC.
Depending on the source α-amylase works best between pH 5–7 and at around 20 ºC.
In can be denatured by raising the temperature.
Enzymes in Conservation: Protease
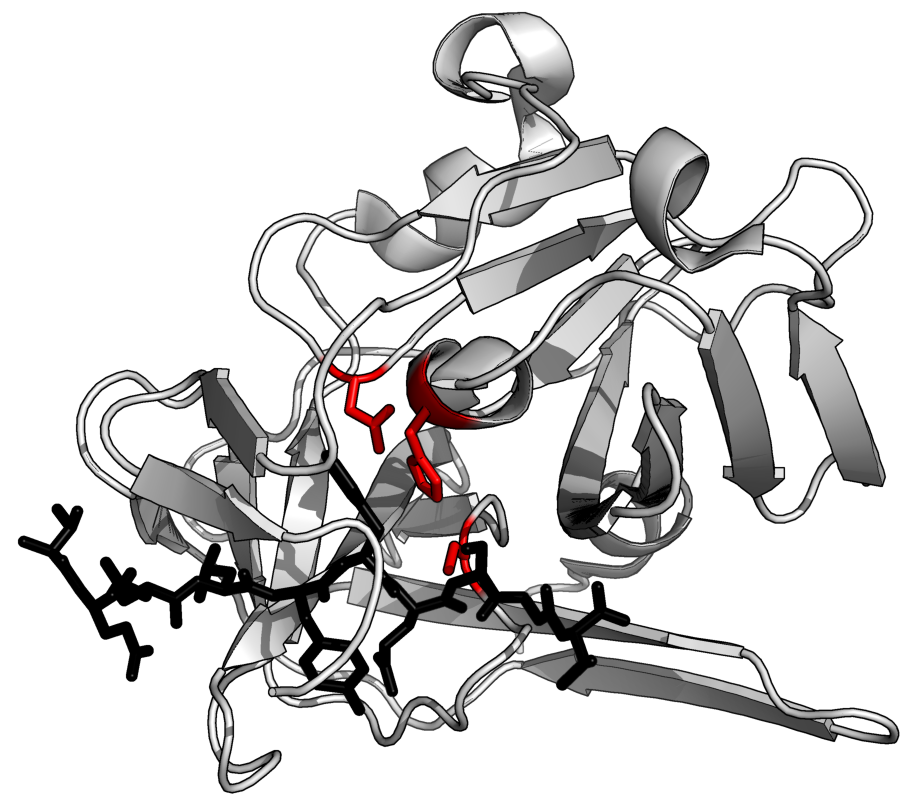 The protease enzymes help to break down the peptide bonds in proteins. Some of the proteases only attack the ends of
the polypeptide chains (exoprotease). Others attack along the chain length (endoprotease). Most commercially available proteases are a mixture of the two.
The protease enzymes help to break down the peptide bonds in proteins. Some of the proteases only attack the ends of
the polypeptide chains (exoprotease). Others attack along the chain length (endoprotease). Most commercially available proteases are a mixture of the two.
Protease: Sources and Conditions
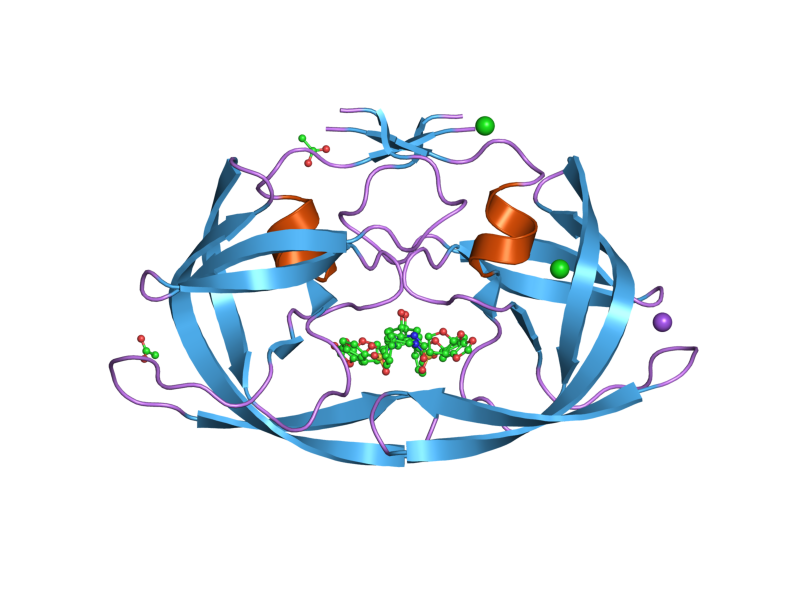 Proteases are found in animals and in several microorganisms such as viruses, protazoa, bacteria, yeast and fungi.
Proteases are found in animals and in several microorganisms such as viruses, protazoa, bacteria, yeast and fungi.
Quite a lot of biochemical research has focused on protease inhibitors in viruses.
Depending on the type of protease and the source, different proteases work between a highly acidic pH and neutral at a temperature of around 37ºC.
Enzymes in Conservation: Lipase

Lipase aids in the hydrolysis of the ester bonds in lipids, usually at the glycerol backbone.
Fats are not water soluble. Lipase helps break them down into hydrophilic alcohols and carboxylic acids.
Lipase Catalyzes Triglyceride Hydrolysis
A triglyceride is a fatty acid ester of glycerol.

Lipase: Optimal Conditions
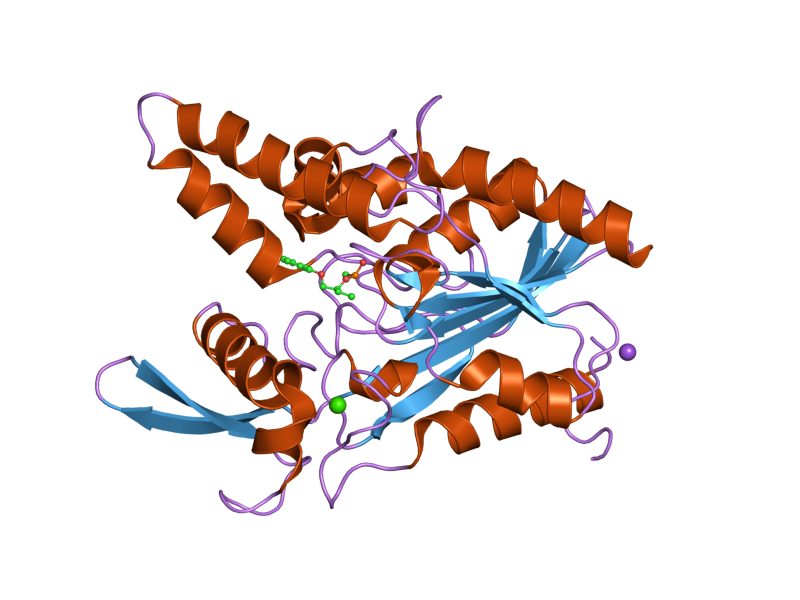
Depending on the type used, lipase works best at a slightly alkaline pH and a temperature around body temperature, 37ºC.
Enzymes in Conservation: Require Optimal Conditions

All enzymes work best in their specific optimal conditions of pH and temperature.
They need to be in some sort of aqueous environment to work. They can be stopped by denaturing them either by:
- Raising the temperature
- Radically altering the pH
Both of these actions will disrupt the hydrogen bonds in the secondary structure.
Enzymes in Conservation: Summary
In this section you learned that:
- Amylases, proteases, and lipases are the three categories of enzymes that are important in art conservation.
- Amylases break down glucose linkages in starch.
- Proteases break down peptide bonds in proteins.
- Lipases break down the ester bonds in lipids.
- Each of these types of reactions helps ceate products that are water soluble.
- Enzymes require the proper pH and temperature to work best.
References
These articles discuss the use of enzymes in conservation. They can all be found at JAIC Online.
Credits
Researched and written by Sheila Fairbrass Siegler
Instructional Design by Cyrelle Gerson of Webucate Us
Project Management by Eric Pourchot
Special thanks to members of the Association of North American Graduate Programs in Conservation (ANAGPIC) and the AIC Board of Directors for reviewing these materials.
This project was conceived at a Directors Retreat organized by the Getty Conservation Institute and was developed with grant funding from the Getty Foundation.
Converted to HTML5 by Avery Bazemore, 2021
© 2008 Foundation for Advancement in Conservation