Enthalpy of
Denaturation and Surface Functional Properties of Heated Egg White
Proteins in the Dry State
AKIO KATO, HISHAM R. IBRAHIM, HIROYUKI
WATANABE, KAZUO HONMA, and KUNIHIKO KOBAYASHI
Authors Kato, Ibrahim, and Kobayashi are with the Dept. of
Agricultural Chemistry, Faculty of Agriculture, Yamaguchi Univ.,
Yamaguchi 753, Japan. Authors Watanabe and Honma are with OP.
Corporation, Fuchu-shi, Tokyo 183, Japan.
Abstract
The enthalpy of denaturation ( H) and surface properties of proteins were
related to elucidate the mechanisms of foaming and emulsifying
properties by using various heated egg white proteins in the dry
state. Foaming and emulsifying properties of all sample proteins
were greatly increased with a decrease in the enthalpy of
denaturation as determined by differential scanning calorimetry
analysis. In plots, foaming and emulsifying properties correlated
linearly with
H) and surface properties of proteins were
related to elucidate the mechanisms of foaming and emulsifying
properties by using various heated egg white proteins in the dry
state. Foaming and emulsifying properties of all sample proteins
were greatly increased with a decrease in the enthalpy of
denaturation as determined by differential scanning calorimetry
analysis. In plots, foaming and emulsifying properties correlated
linearly with  H values for various dry-heated egg white proteins. Thus,
the enthalpy of denaturation of proteins seemed to be a significant
structural factor governing surface functional properties.
H values for various dry-heated egg white proteins. Thus,
the enthalpy of denaturation of proteins seemed to be a significant
structural factor governing surface functional properties.
Introduction
AMPHIPHILIC PROTEINS are principally surface active agents (Kato
and Nakai, 1980; Kinsella, 1981; Halling, 1981). Thus, awareness has
increased on the role of surface hydrophobicity in the functional
properties of proteins. Many studies have focused on the protein
hydrophobicity as a structural factor affecting functional
properties such as emulsification (Kato and Nakai, 1980; Shimizu et
al., 1983) and foaming (Horiuchi and Fukushima, 1978; Townsend and
Nakai, 1983). In addition to protein hydrophobicity, the surface
functional properties are also related to molecular size (Kato et
al., 1985b), conformational stability (Kato and Yutani, 1988), and
net charge (Kato et al., 1987).
Aside from the structural factors, interfacial film formation is
an essential event in formation of foam and emulsion. In foaming
systems the formation of strong cohesive viscoelastic film is
desirable for stable foaming. This may be a case of emulsion
stability. Protein diffuses and adheres to the interface, the
tertiary structure of polypeptide unfolds to a certain extent and
spreads, because of the favorable thermodynamic situation at the
interface. These dynamic events are influenced by the stability of
the tertiary structure of proteins and the predominant environmental
conditions. Recently, Kato and Yutani (1988) proved the importance
of protein stability in surface functional properties using protein
engineering techniques. The surface properties of natural and six
mutant tryptophan synthase substituted at the same position, 49,
were measured by surface tension, foaming and emulsifying properties
and the surface properties were correlated with stabilities. Good
correlations were observed between these surface properties and
values of the Gibbs free energy of proteins unfolding in water. The
parameter of protein stability used in that experiment was
calculated from the folding-unfolding equilibrium state. On the
other hand, the enthalpy of denaturation, reflecting protein
stability, can be determined directly by calorimetry using
differential scanning microcalorimetry. Thus, we were interested in
investigating whether the enthalpy of denaturation is related to
surface properties in order to correlate more closely the structural
and functional properties of proteins.
We reported the improvement of functional properties of egg white
proteins by dry-heating without loss of solubility or deteriorative
effect on conformation. This phenomenon is suitable for studying the
structure-function relation of proteins, because various
conformational states can be obtained using the same protein. In our
present study, various egg white proteins heated in the dry state,
were used to estimate the influence of enthalpy of denaturation
( H) on
their functional properties.
H) on
their functional properties.
Materials & Methods
EGG WHITE (DEW), spray-dried at 60-70°C after decarbohydrate
treatment, was provided by Q.P. Corporation, Tokyo. Heat treatment
of DEW was done as follow; 5g DEW were placed in a test tube,
tightly sealed and incubated at 80°C for various periods of time
(days) in the dry state (7.5% moisture). After heating to the
given time, the tube was removed from the incubator and cooled to
room temperature (25°C); subsequently surface functional
properties were measured. The measurements were performed after
passing the sample solutions through a filter paper to remove
insoluble materials and then protein concentration was adjusted
spectrophotometrically. However, we found the absorbance at 280 nm
of the samples before and after filtration remained essentially the
same.
Foaming properties of heated DEW in the dry state were determined
by measuring the conductivity of foams produced when air at a
constant flow rate of 90 cm2/min was introduced for 15
sec into 5 mL of 0.125% DEW protein concentration in 20 mM phosphate
buffer, pH 7.4, in a vertical glass column (2.4x30 cm) with a glass
filter at the bottom (Kato et al. ,1983). The conductivity of foams
was measured by an electrode with a cell fixed inside the glass
column 1 cm away from and 2.4 cm above the filter, connected to a
conductivity meter (Kyoto Electrics Industry Co., Model CM- 07).
Foaming power was defined as the maximum conductivity of the foams
15 sec after air was introduced. Foam stability was calculated from
conductivity curves as time until the foam was not apparent.
Emulsifying properties of heated DEW in the dry state were
determined by the conductivity method (Kato et al., 1985a). The
emulsions were prepared as follow: 5 mL of corn oil and 15 mL of
0.185% DEW protein solution in 100 mM phosphate buffer, pH 7.4, were
homogenized in Ultra Turax equipment (Hansen and Co., West Germany)
at 12000 rpm for 1 min at 20°C. The emulsifying activity of each
emulsion was calculated from the difference between the conductivity
of protein solution and emulsion. The stability of each emulsion was
calculated from the initial slope of the conductivity curve, as
described previously (Kato et al., 1985a).
The thermal characteristics of egg white proteins heated in the
dry state for various periods were examined by differential scanning
calorimetry (DSC-100, Seiko, thermal analyzer, equipped with a DSC
cell). In a typical experiment, 50 µL of about 10% protein
solution was sealed in a preweighed hermetic aluminum pan and
weighed. Another pan containing water with no protein was used as
the reference. The pans were heated in the calorimeter at a linear
rate of 1°C/min over the range of 30-120°C. The denaturation
temperature (Td) and enthalpy of denaturation ( H) were computed
from the thermograms by the SSC-5000 analyzer (Seiko Electric
Industry Co.).
H) were computed
from the thermograms by the SSC-5000 analyzer (Seiko Electric
Industry Co.).
All tests in this study were performed in duplicate, and the
deviations were minor.
Results & Discussion
OUR PREVIOUS REPORT (Kato et al., 1990) showed the data of
calorimetric analysis for DEW, globulin or albumin fractions and
purified ovalbumin as a function of heating time in the dry state.
The values of  H dramatically decreased with an increase of heating time
in the dry state for all protein samples. The rate of decrease in
H dramatically decreased with an increase of heating time
in the dry state for all protein samples. The rate of decrease in
 H was
dependent on the protein. Reduction in the
H was
dependent on the protein. Reduction in the  H was
accompanied with a slight decrease in denaturation temperature (Td).
This result suggested changes in molecular conformation, i.e.,
unfolding of the tertiary structures as reflected by the marked
decrease in 'H and Td values. Accordingly, the approach was
attempted relate improved surface properties with such thermally
altered molecules.
H was
accompanied with a slight decrease in denaturation temperature (Td).
This result suggested changes in molecular conformation, i.e.,
unfolding of the tertiary structures as reflected by the marked
decrease in 'H and Td values. Accordingly, the approach was
attempted relate improved surface properties with such thermally
altered molecules.

Fig. 1--Foaming properties of egg white
fractions as a function of heating time in the dry state. (A)
foaming power; (B) foam stability. The determination was carried out
for DEW (•), globulin fraction (0), albumin
fraction (0) and purified ovalbumin (A).

Fig. 2--Emulsifying properties of egg white
fractions as a function of heating time in the dry state. A)
emulsifying activity; B) emulsion stability. Symbols as in Fig. 1.
The foaming properties of native and dry heated DEW, globulin or
albumin fractions and purified ovalbumin are shown in Fig. 1. Increased heating time in the dry state
caused a significant increase in both foaming power and foam
stability of all samples. The greatest foaming power was obtained
from heated DEW. The globulin fractions had a higher foaming power
than the albumin fractions or purified ovalbumin. Increase in
heating time in the dry state resulted in increased foam stability
of all samples. Foam stability of DEW was much greater than that of
other samples at any heating time including the non-heated sample.
Foam stability of the native globulin fraction was higher than that
of albumin fraction or purified ovalbumin. These observations
suggested that occurrence of protein-protein interaction might be
facilitated in DEW leading to the formation of strong foam film.
The emulsifying properties of dry heated DEW, globulin or albumin
fractions and purified ovalbumin are shown in Fig.
2.
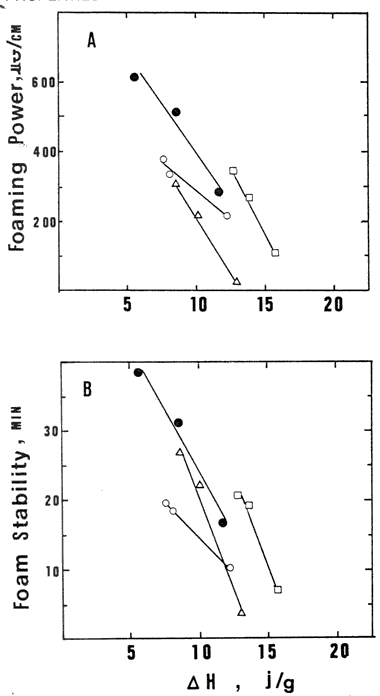
Fig. 3--Plots of foaming properties versus A H of
heated egg white proteins in the dry state for various periods of
time. Key:
(A) foaming power; (B) foam stability. Symbols as in Fig. 1.
With increased heating time in the dry state, marked improvement
in both emulsifying activity and emulsion stability was observed for
all samples. The highest emulsifying activity was obtained from DEW
and the globulin fraction. The emulsion formed from purified
ovalbumin was usually less active compared to those formed from
other fractions. The emulsion stability of the globulin fraction was
much higher than that of DEW, albumin fraction or purified ovalbumin
at any heating time. The emulsion stability of DEW remained higher
than that of albumin fraction. Emulsion stability of purified
ovalbumin was less altered by dry heating than the other egg white
fractions -

Fig. 4--Plots of emulsifying properties versus A H
of heated egg white proteins in the dry state for various periods of
time. Key: (A) emulsifying activity; (B) emulsion stability. Symbols
as in Fig. 1.
Plots of foaming properties and enthalpy of molecular unfolding
for dry heated DEW were constructed to study the contribution of
thermodynamic stability of proteins to surface properties (Fig. 3). Good linear correlations were observed
between decrease in  H and increase in foaming power and foam stability for
the protein constituents of each fraction of egg white. Therefore,
these results indicated a clear relationship between enthalpy of
unfolding values of dry heated DEW proteins and their functionality.
The plots of
H and increase in foaming power and foam stability for
the protein constituents of each fraction of egg white. Therefore,
these results indicated a clear relationship between enthalpy of
unfolding values of dry heated DEW proteins and their functionality.
The plots of  H vs. emulsifying properties of dry heated DEW proteins
are shown in Fig. 4. Similarly, linear
correlation between
H vs. emulsifying properties of dry heated DEW proteins
are shown in Fig. 4. Similarly, linear
correlation between  H and emulsifying activity and emulsion stability were
observed. Although linear correlation between
H and emulsifying activity and emulsion stability were
observed. Although linear correlation between  H and emulsion
stability of ovalbumin was observed, it was quite deviated from the
regression line. This behavior of ovalbumin was presumed to be
predominantly due to its amphiphilic nature since this structural
element is well known to have a critical role in emulsion stability.
Also it may be that multi-protein components of egg white are
required to attain strong correlations between
H and emulsion
stability of ovalbumin was observed, it was quite deviated from the
regression line. This behavior of ovalbumin was presumed to be
predominantly due to its amphiphilic nature since this structural
element is well known to have a critical role in emulsion stability.
Also it may be that multi-protein components of egg white are
required to attain strong correlations between  H and
emulsifying properties of DEW as a function of heating time in the
dry state. However, the decrease in enthalpy of denaturation of the
proteins by dry heating appeared to be a major contributor to the
significant improvement of foaming and emulsifying properties.
H and
emulsifying properties of DEW as a function of heating time in the
dry state. However, the decrease in enthalpy of denaturation of the
proteins by dry heating appeared to be a major contributor to the
significant improvement of foaming and emulsifying properties.
Our approach indicates that  H is a determinant of foaming and emulsifying
properties. Our previous studies revealed that heating of DEW in the
dry state caused a substantial increase in its molecular flexibility
(Kato et al., 1990) and surface hydrophobicity (Kato et al., 1989).
In addition, circular dichroism spectra of dry heated DEW indicated
very slight conformational changes. Our experimental results enable
thermodynamic considerations for surface and functional properties
proteins. The reduction of
H is a determinant of foaming and emulsifying
properties. Our previous studies revealed that heating of DEW in the
dry state caused a substantial increase in its molecular flexibility
(Kato et al., 1990) and surface hydrophobicity (Kato et al., 1989).
In addition, circular dichroism spectra of dry heated DEW indicated
very slight conformational changes. Our experimental results enable
thermodynamic considerations for surface and functional properties
proteins. The reduction of  H of egg white proteins by heating may have
enhanced the rate of diffusion of the thermally altered molecules to
the interface, facilitating protein-protein association in formation
of interfacial films. This would not be surprising, since Kato et al
(1990) found definitive experimental proof of a strong relationship
between enthalpy of denaturation and gel strength. High foaming and
emulsifying properties of dry heated proteins reflect faster
unfolding and greater intermolecular interaction at the interface.
Proteins having high entropy due to reduction of AH and more
flexible polypeptide chains should facilitate a greater degree of
association by hydrophobic and electrostatic interactions. The high
foam stability of dry heated proteins reflected greater molecular
interaction to form more cohesive film. This film comprised
extensive overlapping of coiled polypeptides, which allowed it to
expand upon application of stress.
H of egg white proteins by heating may have
enhanced the rate of diffusion of the thermally altered molecules to
the interface, facilitating protein-protein association in formation
of interfacial films. This would not be surprising, since Kato et al
(1990) found definitive experimental proof of a strong relationship
between enthalpy of denaturation and gel strength. High foaming and
emulsifying properties of dry heated proteins reflect faster
unfolding and greater intermolecular interaction at the interface.
Proteins having high entropy due to reduction of AH and more
flexible polypeptide chains should facilitate a greater degree of
association by hydrophobic and electrostatic interactions. The high
foam stability of dry heated proteins reflected greater molecular
interaction to form more cohesive film. This film comprised
extensive overlapping of coiled polypeptides, which allowed it to
expand upon application of stress.
Our study is clearly broadening our concept of the relationship
between thermodynamics and foam or emulsion forming ability of egg
white proteins. Generally we can say that foams and emulsions from
dry heated proteins were more stable than those from native
proteins. Foaming and emulsifying properties were greater when the
enthalpy of denaturation of these proteins was lowered by dry
heating.
In conclusion, our observations indicate that the formation and
the properties of interfacial films were mainly attributed to
thermodynamic characteristics of the proteins. Further studies are
needed to determine whether these relationships are applicable for
other proteins of different food sources as well.
References
Halting, P.J. 1981. Protein stabilized foam and
emulsions. CRC Crit. Rev., Food Sci. Nutr. 15: 155.
Horiuchi, T. and Fukushima, D .1978. Studies on
enzyme-modified proteins as foaming agents: Effect of structure on
roam stability. Food chem. 3:35.
Kato, A., Fujishige, T., Matsudomi, N., and
Kobayashi, K. 1985a. Determination of emulsifying properties of some
proteins by conductivity measurements. J. Food Sci. 50: 56.
Kato, A., Hisham, R.I., Watanabe, H., Honma, K.,
and Kobayashi, K.1989. New approach to improve gelling and surface
functional properties of dried egg white by heating in dry state. J.
Agric Food Chem. 37: 433.
Kato, A., Hisham, R.I., Watanabe, H., Honma, K.,
and Kobayashi, K.1990. Structural and gelling properties of dry
heated egg white proteins. J. Agric. Food Chem. 38: 32.
Kato, A., Komatsu, K., Fujimoto, K., and
Kobayashi, K. 1985b. Relationship between surface functional
properties and flexibility of proteins detected by the protease
susceptibility. J. Agric. Food Chem. 33: 931.
Kato, A., Miyachi, N., Matsudomi, N., and
Kobayashi, K.1987. The role of sialic acid in the functional
properties of ovomucin. Agric. Biol. chem. 51: 641.
Kato, A. and Nakai, S. 1980. Hydrophobicity
determined by a fluorescence probe method and its correlation with
surface properties of proteins. Biochem. Biophys. Acta. 624: 13.
Kato, A., Takahashi, A., Matsudomi, N., and
Kobayashi, K. 1983. Determination of foaming properties of proteins
by conductivity measurements. J. Food Sci. 48: 62.
Kato, A. and Yutani, K. 1988. Correlation of
surface properties with conformational stabilities of wild-type and
six mutant tryptophan synthase alfa-subunits substituted at the same
position. Protein Eng. 2: 153.
Kinsella, J.E. 1981. Relationship between
structure and functional properties of food proteins. In Food
Proteins. P.F. Fox and ,J.J. Condon (Ed.). Applied Science
Publishers, London and New York.
Shimizu, M., Takahashi, T., Kaminogawa, S., and
Yamauchi, K. 1983. Adsorption onto an oil surface and emulsifying
properties of bovine  sl casein in relation to its molecular
structure. J. Agric Food Chem. 31:1214.
sl casein in relation to its molecular
structure. J. Agric Food Chem. 31:1214.
Townsend, A.A. and Nakai, S. 1983. Relationships
between hydrophobicity and foaming characteristics of food proteins.
J. Food Sci. 48: 588.
Ms received 9/8/89; revised 12/2/89; accepted
3/6/90.
 H) and surface properties of proteins were
related to elucidate the mechanisms of foaming and emulsifying
properties by using various heated egg white proteins in the dry
state. Foaming and emulsifying properties of all sample proteins
were greatly increased with a decrease in the enthalpy of
denaturation as determined by differential scanning calorimetry
analysis. In plots, foaming and emulsifying properties correlated
linearly with
H) and surface properties of proteins were
related to elucidate the mechanisms of foaming and emulsifying
properties by using various heated egg white proteins in the dry
state. Foaming and emulsifying properties of all sample proteins
were greatly increased with a decrease in the enthalpy of
denaturation as determined by differential scanning calorimetry
analysis. In plots, foaming and emulsifying properties correlated
linearly with  H values for various dry-heated egg white proteins. Thus,
the enthalpy of denaturation of proteins seemed to be a significant
structural factor governing surface functional properties.
H values for various dry-heated egg white proteins. Thus,
the enthalpy of denaturation of proteins seemed to be a significant
structural factor governing surface functional properties.




 sl casein in relation to its molecular
structure. J. Agric Food Chem. 31:1214.
sl casein in relation to its molecular
structure. J. Agric Food Chem. 31:1214.