
Poultry Science. Vol. 60. 1981 pp. 2071-2083
(Received for publication November 13, 1980)
Published as Michigan Agricultural Experiment Station Journal Article No. 9713. This research was supported, in part, by a fellowship from the American Egg Board to Dr. Johnson, presently affiliated with F.M.C. Corporation, Lakeland, FL 33802.
(Key words: albumin gels, protein interactions, globulins, ovalbumin, ovomucoid, ovomucin, conalbumin, lysozyme)
Studies of gelation properties of the albumen proteins in a custard model system showed that conalbumin was the least heat stable protein with a denaturation temperature of 57.3 C. Globulins and ovalbumin ranked second with denaturation transition temperatures of 72.0 and 71.5 C, respectively. Lysozyme denatured at 81.5 C while ovomucin and ovomucoid showed no coagulation abilities. Lysozyme produced the strongest gel followed by globulins, ovalbumin, and conalbumin.
In a study of binary mixtures, aggregation of polypeptides occurred near the denaturation transition temperature of the least heat stable protein. Therefore, in combinations of lysozyme, globulins, and ovalbumin with conalbumin, denaturation was apparent at 58.3, 57.8, and 58.1 C, respectively. Ovomucoid consistently increased the coagulation temperature ranges of globulins, conalbumin, and ovalbumin, and prevented coagulation of lysozyme. Gel strength varied according to the proteins present.
The combinations of five or all proteins resulted in more subtle changes of temperature during coagulation. The destabilizing effects of conalbumin on the other proteins were still apparent. The control mixture exhibited two distinct coagulation temperature ranges of 61.5 to 62.5 C and 73.0 to 71.0 C.
In the scanning electron microscopic investigations of selected coagulums it was found that lysozyme, conalbumin, and ovalbumin polypeptides aggregated in grape-like clusters of variable size. The control mixture gel also exhibited the same pattern. Globulins polypeptides appeared to tightly associate in membrane-like arrangements and showed excellent binding abilities. Small duster sizes seemed to parallel gel firmness.
Protein gels are composed of three-dimensional matrices of partially associated polypeptides with water held in the interstices (Kinsella, 1976). Ferri (1948) described gelation as a two-stage process initiated by heat denaturation of the protein molecules into unfolded polypeptides and then association of the polypeptides forming the gel matrix. The interactions may involve hydrogen bonds, disulfide bonds, hydrophobic association, or a combination of these (Catsimpoolas and Meyer, 1970). Studies on gelation properties indicate many proteins have the capacity to form gels upon heat treatment. These include soy proteins (Saio et al., 1974), fish protein (Hermansson and Akesson, 1975), leaf protein (Lu and Kinsella, 1972), sunflower, fababean, and field pea proteins (Fleming et al., 1975). Protein concentration, pH, salts, sugar, lipids, and temperature all affect firmness and characteristics of protein gels (Catsimpoolas and Meyer, 1970; Saio et al., 1974; Fleming et al., 1975; Lu and Kinsella, 1972).
In studies of coagulation ability of egg products several factors such as salt, sugar, acid, alkali, and temperature have been listed as the major parameters influencing the heat induced gelling of eggs (Baldwin, 1977). The different heat sensitivity of albumen proteins (Cunningham and Lineweaver, 1965) suggests that some proteins may play major roles in defining the gelling ability of egg white.
The effects of protein to protein interactions on coagulation abilities should be evaluated in relation to performance in food systems. The objective of the present study was to evaluate the possible interactions among the albumen proteins in a custard model system.
Protein Fractionation. The procedure utilized for obtaining the various proteins has been described previously (Johnson and Zabik, 1981).
Determination of sulfhydryl groups was performed as described by Johnson and Zabik (1981) except that samples were dissolved in 5 ml of the buffer solution containing 2% sodium dodecyl sulfate. Solutions with slight cloudiness were filtered through Whatman filter paper No. 4 before absorbance measurement.
Gel Preparation. To prevent any possible interactions among milk, yolk, and albumen proteins, as well as visual interferences at the ultrastructural level, yolk and milk were eliminated from the system. Additional egg white proteins were used to replace the yolk proteins and a salt solution with a similar ionic strength (.165) substituted for milk. This solution was prepared according to the milk mineral composition listed in the Michigan State University Nutrient Data Bank (1978). The salts used were calcium, sodium, magnesium, potassium, and ferric, all in the chloride form. This model system had protein levels similar to that of a custard and as such additional salts were necessary for gelation. The effects of milk proteins and sugar should be studied in subsequent investigations. Duplicate gels were prepared per treatment.
The formula used to prepare the gels consisted of a 1:4 mixture of protein solution:salt solution, resulting in a 1.27% protein concentration in the final mixture. A total of .78 g of proteins was dissolved in deionized water with added sodium and calcium chloride solutions to reconstitute the equivalent of 6.0 g of whole egg (13% protein, liquid basis). To this mixture was added the salt solution replacing milk, and with constant stirring, the pH was adjusted to 8.0 with 1 N potassium hydroxide solution. The resulting solution was transferred to a 50 ml beaker that had been previously sprayed with silicone to prevent sticking of the gel to the container walls. The beaker was covered with a double layer of Saran® wrapping film and placed in a 10 x 10 x 4.5 cm pan filled with an 8% sodium chloride solution. This salt solution was used as the bath medium to prevent excessive evaporation of the water during heating.
The heating system consisted of two Corning Hot Plate Stirrer units, model PC-351, each connected to a Staco Variable Autotransformer, type 2PF 1010, set to 80. The temperature of the protein solution was monitored with a thermocouple securely positioned into the protein solution to a depth of .5 cm from the surface. Temperature was recorded in a Honeywell Brown Electronik Potentiometer, model 153 x 65-P12H-II-III-81-A8, modified for two thermocouple terminals to shorten the elapsed time between each temperature recording to 15 sec intervals. The heating rate during coagulation of the various protein solutions, was approximately .74 C/min. Gels prepared with single proteins were heated for 10 min beyond coagulation, while gels with mixtures of proteins were heated to an end temperature of that equivalent to the gelation. temperature of most heat resistant protein in -the mixture. The prepared gels were covered with food wrapping film and stored at 2 to 4 C for further determinations.
Gel Strength Determination. Gel strength was determined the day following gel preparation using an Instron Universal testing instrument, floor model TT-BM. An Instron tension load cell, model D30-36, was adapted for the measurements since a compression load cell of equivalent sensitivity was not available. A probe was made with a 100 g reference weight and a microsyringe glass plunger of .35 cm diameter and 6.8 cm long.
Force resistance curves of the gels plunged with the devised probe were obtained at a cross-head speed of 5 cm/min with the gear shift set to high. After reaching room temperature, the sample was placed on the platform. attached above the cross-head and slowly raised towards the probe until 1.0 cm of the plunger penetrated the gel. At this point the cross-head, was stopped and immediately lowered. Force; curves were obtained from two opposite locations on the gel at a chart speed of 20 cm/min Gel strength was determined from area under the curve and expressed as the work, in grams times centimeters required to plunge through 1 cm of the sample.
Percentage Drainage. Following gel strength determination, the coagulums were carefully removed from the beakers onto a preweighed dish set-up devised for collecting the drained liquid. This set-up consisted of a medium size weighing boat to which a copper wire screen with 10 cm of diameter and seven openings/cm was molded. A Whatman filter paper No. 4 of 11.0 cm of diameter was then placed over the screen. The dishes containing the gels were covered with stainless steel bowls and the liquid allowed to drain for 1 hr. After this period the total gel weight and the liquid weight were determined. The percentage drainage was calculated by dividing the liquid weight by the sample total weight and multiplying by 100.
Small gel portions of approximately 1 cm on a side were frozen in dry ice before the liquid was drained and further freeze dried (Johnson and Zabik, 1981) for sulfhydryl determinations and sample preparation for examination in a scanning electron microscope.
Scanning Electron Microscopy. Small pieces of freeze-dried samples were mounted on aluminum stubs and gold coated with a sputter coater for six to nine minutes. The prepared samples were examined in a Japan Electron Optics Limited SEM, model JSM 35C, at an accelerating voltage of 15kV.
pH Determination. The protein coagulum and drained liquid obtained from the previous determination were combined, and the curd was broken into small pieces with a metal spatula until a slurry was formed. The pH of this mixture was measured on a Beckman Expandomatic pH meter, model 76A.
Experimental Design. Each protein was allowed to vary from 0 to 100% and tested individually and in combinations of two or five proteins. The control gel was prepared with the normal adjusted levels of the proteins as found in egg white. Table 1 lists the treatments used for preparing the gels.
Statistical Analysis. Gel data were analyzed by the Student's t statistics for comparison of two means with unknown but equal variances (Gill, 1978).
a Levels listed by Powrie (1977) adjusted to a total of 100%
FIG. 1. Time-temperature curves of several albumen protein solutions heated at a rate of .74 C/min. Protein concentration=1.27%, ionic strength=.275, ph=8.
The time-temperature relationships for gels prepared with lysozyme, globulins, conalbumin, and ovalbumin are illustrated in Figure 1. A rapid increase in temperature followed by a decrease and leveling off during heating was observed for all gels. The decrease in temperature marked the onset of coagulation and also showed that aggregation of the polypeptides proceeded with absorption of energy from the system (endothermic process).
a,b,c,d,e,f,g The t test was used for comparison of means for initial coagulation temperature, gel strength, and drainage. Values within a column with the same superscript are significantly different at P<.05.
1 Duplicate determinations.
2 Protein concentration=1.27%, initial ph=8.0, ionic strength .275; mixtures are 1:1 on a weight basis,
3 nc=no coagulation.
4 nd=not determined.
5 Values with no standard deviation are estimated from individual proteins sulfhydryl content.
The physicochemical characteristics of gels prepared with the various proteins are shown in Table 2. Conalbumin coagulation initiated at 57.3 C and aggregation ensued, promoting a drop of 1.3 C in temperature. Ovalbumin coagulation temperature ranged from 71.5 to 71.0 C. Aggregation of the polypeptides proceeded with a modest drop of .5 C in temperature. A temperature range of 72.0 to 69.9 C for coagulation of globulins was observed with a drop of about 2 C in temperature. Lysozyme showed the highest thermal requirement for unfolding of the polypeptides, 81.5 C. Formation of the coagulum ensued with a drop of 7 C in temperature. The initial coagulation temperatures were significantly different among the various gels except for ovalbumin and globulins gels.
Ovomucin and ovomucoid showed no gelation properties under the conditions used for evaluations. The observed heat stability of these proteins is in close agreement with the earlier findings of MacDonnell et al. (1953) and Lineweaver and Murray (1947) for ovomucin and ovomucoid, respectively.
Significant differences among the various protein gel firmness were observed (Table 2). Lysozyme produced the firmest gel (43.98 g x cm) of all proteins, followed by globulins (14.26 g x cm), ovalbumin (5.68 g x cm) and conalbumin (3.61 g X cm). Lysozyme coagulum showed the least loss of liquid. Globulin and ovalbumin coagulums showed similar percentage drainage. The highest loss of liquid was exhibited by conalbumin gel. Significant differences between percentage drainages of lysozyme and globulins gels and of lysozyme, and conalbumin gels were observed.
The great variability in temperature for initiation of denaturation observed for the various proteins is apparently associated with protein molecular conformation. Disulfide, as well as hydrogen, hydrophobic, and electrostatic bonds, and Van der Waals attractions stabilize the protein structure. The covalent SS linkage has fairly high heat of formation ranging from 30 to 100 kcal/mole (Whitaker, 1977). Consequently, disruption also requires fairly high energy. Ionic attractions rank second with approximately 10 to 20 kcal/mole, followed by hydrogen bonds, 1 to 5 kcal/mole and Van der Waals attractive forces with 1 to 3 kcal/mole.
Proteins that are compact in shape as well as bearing several disulfide bonds appear to show higher resistance to heat denaturation (Whitaker, 1977). Therefore, the relatively high denaturation temperature of lysozyme (81.5 C) may have resulted from its nearly spherical shape and the high concentration of SS groups, 2.74 moles/104 g protein (Johnson and Zabik, 1981), maintaining the protein structure. Nevertheless, the rank order of SS groups o globulins, ovalbumin, and conalbumin, 1.51, .30, and 1.85 mole/104 g proteins, do not correspond to their respective ranks of resistance to heat denaturation.
Formation of stronger gels may be related to more extensive crosslinking of the polypeptides. The drastic drop in temperature of 7 C during lysozyme coagulation paralleled by formation of the firmest gel suggests more extensive SS linkages were involved in the aggregation phenomenon of this protein. Sulfhydryl disulfide interchange has been reported-to be responsible for heat aggregation of proteins (Kinsella, 1976)-
Protein Interactions. The binary mixtures of lysozyme, globulins, ovomucoid, conalbumin, and ovalbumin had significant effects on coagulation temperature, gel strength, and percentage drainage of the coagulums (Table 2). The changes observed in the coagulation temperature, firmness, and percentage drainage with these double protein combinations are illustrated in Figure 2. Lysozyme-ovomucoid combination did not coagulate, suggesting that ovomucoid had a protective effect on lysozyme, thus preventing denaturation (Table 2). The extent of the effects on lysozyme gel coagulation temperature and firmness was pronounced, whereas percentage drainage was moderately affected. Globulins, conalbumin, and ovalbumin reduced coagulation temperature and gel strength and increased liquid loss (Figure 2A).
A) Lysozyme and combinations
B) Globulins and combinations
C) Conalbumin and combinations
D)Ovalbumin and combinations
FIG. 2. A comparison of changes in coagulation
temperature, gel firmness, and percentage drainage of various
protein coagulums, LYS—lysozyme, GLOB — globulins, CON
— conalbumin, OVB—ovalbumin, and OVD—ovomucoid.
Protein concentration=1.27%, ph=8, ionic strength .275.
A) Lysozyme and combinations;
B) Globulins and combinations;
C) Conalbumin and combinations;
D)Ovalbumin and combinations.
Similar trends were observed for the effects of double combinations in the other protein gels (Figure 2B, C, and D). Ovomucoid raised the denaturation temperature of the other proteins, but gel strength decreased considerably. It appeared that ovomucoid did not participate in the aggregation phenomena leading to formation of weaker gels, which was also reflected in the poor liquid holding ability of the gel.
Conalbumin and its combinations consistently showed the lowest coagulation temperature. Lysozyme and globulins double combinations showed the highest gel strengths which indicated that these proteins were actively involved in development of the coagulums.
In general. heating decreased the number of SH groups of the coagulums (Table 2). Significant reductions in SH groups of globulins, ovalbumin, and the combination of globulins and ovalbumin gels were observed. A significant decrease in pH from 8.00 to 7.58 (treatment total) occurred with heating. This indicated that more acidic groups were exposed with the treatment.
FIG. 3. Comparison of time-temperature curves of various solutions with different protein composition. Heating rate=.74 C/min, protein concentration=1.27%, ph=8, ionic strength=.275.
FIG. 4. Comparison of time-temperature curves of solutions with different protein composition. Heating rate=.74% C/mm, protein concentration=1.27%, ph=8, ionic strength=.275.
The time-temperature relationship of the gels containing five and six proteins are illustrated in Figures 3 and 4. The mixture containing all proteins showed a slight decrease in temperature (.5 C) after about 15 min of heating followed by a fairly rapid increase in temperature (Figure 3). The mixture lacking ovomucin coagulated at two different temperatures after about 23 and 38 min of heating. The other protein mixtures did not show an apparent drop in temperature during coagulation, but the slight decrease in rate of heat penetration, seen in the curves, marked the onset of coagulation.
The mixture with no globulins showed a slight drop in temperature during coagulation (Figure 4). The mixture lacking conalbumin coagulated after about 25 min of heating, whereas without ovalbumin, coagulation took place earlier.
a,b,c,d,e,f,g Values within a column with different superscripts are significantly different at P<.05.
1 Values are average of two determinations.
2 Protein concentration = 1.27%, initial pH = 8, ionic strength = .275.
3 Levels of the proteins in mixtures of 5=20%, in mixtures of 6=16.6%; ON—ovomucin, LYS—Lysozyme, GLOB—globulins, OVD—ovomucoid, OVB— ovalbumin, CON—conalbumin.
4 Estimated values.
The characteristics of the gels prepared with the protein mixtures are summarized in Table 3. Mixtures containing ovomucin, lysozyme, and conalbumin simultaneously showed an initial coagulation temperature ranging from 55 to 59 C. Removal of conalbumin raised the coagulation temperature range to 65 to 75 C. Similar effect was observed with the mixture lacking lysozyme. Omitting ovomucin induced coagulation at two temperatures, 59.4 to 58.9 C and 71.3 to 70.8 C. The control also displayed two coagulation temperature ranges, 61.5 to 62.5 C and 73.0 to 71.0 C. The first coagulation temperature range indicated conalbumin and possibly some other protein(s) denatured with partial aggregation, and coagulation was completed at the second temperature range.
Firmest gels were produced with the mixtures with no ovalbumin (12.17 g x cm) and no ovomucoid (10.48 g x cm). Control and gel with no ovomucin ranked second, with values of 6.34 and 6.73 g X cm, respectively.. Gels with no globulins (597 g x cm) and gels with all proteins (5.54 g x cm) ranked third, followed by the gel with no lysozyme (4.36 g x cm) and the gel with no conalbumin (3.79 g x cm).
In general, gels containing ovomucin in the combination of five proteins exhibited better liquid retention with percentage drainage values ranging from 0 to 62%. No liquid drained from gel lacking lysozyme. Evidently ovomucin retained most of the liquid in the swollen carbohydrate moieties. The gel with no ovomucin had the highest liquid loss (66%) followed by the gel containing all proteins (65%). The control gel lost about 61% liquid.
The pH of the gels decreased during heating. The sulfhydryl content of the coagulums was consistently lower than that of the solutions. This supports the idea of SH-SS interchange reactions involvement in cross-linking of the polypeptides.

| 
|

| 
|

| |
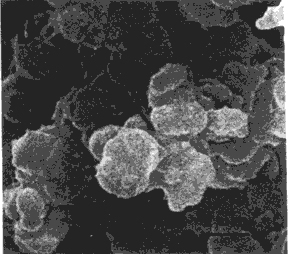
| FIG. 5. Scanning electron micrographs of albumen proteins gel. A) Lysozyme, B) Globulins, C) Conalbumin, D) Ovalbumin, E) Control. | |

| 
|

| 
|

| |
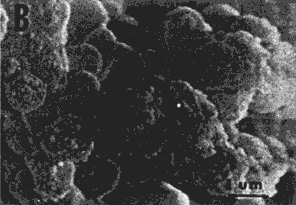
| FIG. 6. Scanning electron micrographs of albumen proteins gel. A) Lysozyme, B) Globulins, C) Conalbumin, D) Ovalbumin, E) Control. | |

| 
|

| 
|
| FIG. 7. Scanning electron micrographs of albumen proteins mixtures gels. A) and B) Lysozyme-globulins, C and D) Ovomucin-Lysozyme-globulins-conalbumin-ovalbumin. | |
Electron Microscopy. Scanning micrographs of selected albumen protein gels are shown in Figures 5 and 6. At the lowest magnification (Figure 5), lysozyme, conalbumin, ovalbumin, and control gels were similar in appearance) displaying a disorganized aggregation of the polypeptide chains. Globulins gel was remarkably different and showed a definite orientation of the cross-linked polypeptides into multiple parallel membranes which were interconnected by numerous projections.
At the next level of magnification (Figure 6) the disorganized arrangement of the polypeptides aggregates in lysozyme, conalbumin, ovalbumin, and control gels appeared to be grape-like clusters. Moreover, differences in cluster size were noticeable in the various gels. Lysozyme gel (micrograph A) was characterized by a finer gel network of relatively small aggregates. These gels possessed the greatest gel strength but intermediate drainage values. Conalbumin and ovalbumin gels were roughly similar and showed mostly larger conglomerates. These gels exhibited the lowest gel strengths. The control gel exhibited clusters of intermediate size range, whereas globulins gel had the smallest polypeptide aggregates very tightly associated in the membrane-like arrangement. The gel containing globulins had the second highest gel strength value.
The scanning electron micrographs of gel containing albumen protein mixtures are shown in Figure 7. Figure 7A and B illustrate lysozyme-globulins combination gel at two levels of magnification. Globulins exhibited the same trend in the orientation of the unfolded polypeptides with occasional inclusion of lysozyme clusters (compare with Figure 6A and B). At higher magnification it was clearly seen that lysozyme clusters were larger in size (compare with Figure 6A).
The striking difference in size of the lysozyme aggregates appeared to be related to its lower coagulation temperature in the presence of globulins. McKenzie et al. (1963) pointed out that gelation may occur before a large number of polypeptide chains are unfolded and when this happens a coarser gel network forms. The gel containing lysozyme and globulins was intermediate in gel strength being weaker than the lysozyme gel but strong within the globulins gel. Drainage, however, was greater than for either of these single protein gels.
Figure 7C and D displays the gel prepared with five proteins (ovomucoid omitted). There were several protein strands which appeared to be ovomucin held by aggregates of polypeptides (Figure 7C). A few large clusters of denatured proteins (arrows) were noticeable. At higher magnification (Figure 7D) the disorganized arrangement of the polypeptides in the gel matrix was evident. It seemed as though globulins predominated in the gel. The coagulated protein appeared to be binding, and in some instances, enveloping the other protein aggregates. These were relatively small in size and the overall appearance of the gel matrix suggested a stronger structure. This observation was consistent with gel strength determination.
Baldwin, R. E., 1977. Functional properties in foods. Page 246-277 in Egg science and technology. W.J. Stadelman and D. J Cotterill, ed. Publ. Avi Co., Inc., Westport, CL
Catsimpoolas, N., and E. W. Meyer, 1970. Gelation phenomena of soybean globulins. 1. Protein-protein interactions. Cereal Chem. 47:559-570.
Cunningham, F. E., and H. Lineweaver, 1965. Stabilization of egg-white proteins to pasteurizing temperatures above 60°C. Food Technol. 19:136-141.
Ferri, J. D., 1948. Protein gels. Adv. Prot. Chem. :1-78.
Fleming, S. E., F. W. Sosulski. and N. W. Hamon, 1975. Gelation and thickening phenomena of vegetable protein products. 3. Food Sci. 40: 805-807.
Gill, 3. L., 1978. Design and analysis of experiments in the animal and medical sciences. Vol. 1. Iowa State Univ. Press, Ames, IA.
Hermansson, A. M., and C. Akesson, 1975. Functional properties of added proteins correlated with properties of meat systems. Effect of concentration and temperature on water-binding properties of model meat systems. J. Food Sci. 40:595-602.
Johnson, T. M., and M. E. Zabik 1981. Egg albumen proteins interactions in an angel food cake system. J. Food Sci.
Kinsella, J. E., 1976. Functional properties of proteins in foods: a survey. Crit. Rev. Food Technol. 7: 2 19—280.
Lineweaver, H., and C. W. Murray, 1947. Identification of the trypsin inhibitor of egg white with ovomucoid. J. Biol. Chem. 171:565-581.
Lu, P. S., and J. E. Kinsella, 1972. Extractability and properties of protein from alfalfa leaf meal. J. Food Sci. 3 7:94-99.
MacDonnell, L. R., C. A. Knight, and R. E. Feeney, 1953. Ovomucin and the antihemagglutinin activity of egg white. Fed. Proc. 12:241.
McKenzie, H. A., M. B. Smith, and Z. C. Wake, 1963. The denaturation of proteins. I. Sedimentation, diffusion, optical rotation, viscosity, and gelation in urea solutions of ovalbumin and bovine serum albumin. Biochem. Biophys. Acta 69: 222-239.
Michigan State University Nutrient Data Bank. 1978. Dept. Food Science Human Nutr., Michigan State University, East Lansing, Ml.
Powrie, W. D., 1977. Chemistry of eggs and egg products. Page 65-91 in Egg science and technology. W.J. Stadelman and 0.J. Cotterill, ed. Avi Publ. Co., Inc., Westport, CT.
Saio, K., I. Sato, and T. Watanabe. 1974. Food uses of soybean 7S and 11S proteins. High temperature expansion characteristics of gels. J. Food Sci. 39:777-782.
Whitaker, J. R., 1977. Denaturation and renaturation of proteins. Page 14-49 in Food proteins. J. R. Whitaker and S. R. Tannenbaum, ed. Avi Publ. Co., Inc., Westport, CT.