*Director, Picture Conservation Division, Public Archives of Canada, 395 Wellington Street, Room B145, OTTAWA, Ontario. Canada, K1A 0N3
**Former temporary staff members in the Picture Conservation Division. Correspondence should be addressed to K.B. Hendriks
Of the principal components of many historical and most contemporary processed photographic materials, gelatin functions as a binding medium that holds the image-forming substance in place. Its stability and preservation should be of interest to keepers of photographic collections. There are many possible methods to evaluate its properties, but none appears to be common in modern conservation laboratories. This contribution reviews some of the available literature on the properties of coated gelatin layers and examines methods for the measurement of these properties. It also reports the results of some experiments on the stability of gelatin layers in different types of historical and contemporary photographs.
Gelatin is a common component in archives and library records, most notably in photographic materials. Connoisseurs of the history of papermaking know gelatin as a size, used widely in the 18th and early 19th century. Besides playing a major role in the food industry (i.e. in the manufacture of gelatin desserts), gelatin was used since the turn of the century almost exclusively as the binding medium and protective colloid for the image-forming substance in photographic records produced by methods that are based on the light sensitivity of silver halides. The latter nowadays consists generally of finely divided elemental silver particles ("silver gelatin images"), or organic dyes. However, in the 19th century carbon was used in so-called Woodburytypes and carbon prints, which also contain a gelatin layer as the binding agent. Most silver gelatin photographic materials have an Interlayer between support and binding medium that may serve various purposes, from functioning as an adhesive between the two layers, to improving picture quality. In most types of photographic papers this interlayer, called baryta layer, consists of barium sulfate in gelatin. In addition to the support (for the most part glass, paper or plastic film) and the above-mentioned image-forming substances, the coated gelatin layer may become the limiting factor for the stability of a given photographic record.
The manufacture of gelatin and its properties are broadly discussed in a monograph by Ward and Courts.1 Being used for the first time in 1871 by R.L. Maddox to make a gelatino-bromide emulsion, its application in photography was summarized in 1923 by Sheppard.2 A classical paper on emulsion making was published by Carroll in 1931.3 More recently, photographic gelatin was discussed by Pouradier,4 and Hill,5 who also published formulae for the preparation of photographic emulsions on a laboratory scale,6 and discussed the literature of gelatin7. In a series of articles published during the 1930s, Sheppard and coworkers from the research laboratories of the Eastman Kodak Company discussed the properties of gelatin sols and gels. 8,,9,10 These papers remain principal sources of information today.
Possible approaches to assess the effect of processing solutions or environmental factors such as temperature, and relative humidity on the stability of coated gelatin can be grouped into three categories:
and of coated gelatin layers:
Methods for the testing of physical characteristics have been reviewed by Current.13 Chemical analyses, even when performed in fully automated analytical equipment, are too complex and time-consuming to be carried out on large numbers of samples. One can monitor the chemical changes in the dyes of a faded colour photograph by measuring physical changes, such as density values; similarly, by determining changes in physical properties of gelatin, such as a decrease in the mushiness value, it is possible to infer chemical changes in the gelatin. For purposes of determining the influence of gelatin layers on the stability of processed photographic films, samples were reported to have been incubated at 1000 C in a dry oven, as well as at a temperature of 71° C, and a relative humidity of 50%.11 The results show that the stability of the gelatin is influenced by the type of support onto which it is 'coated. The results of experiments to examine the Physical properties of unsupported gelatin layers have also been reported. 2 Generally speaking, gelatin layers have a longevity comparable to that of the cellulose triacetate onto which they are coated, and are quite stable as long as they are kept dry.14 Gelatin withstands dry heat at 100°C for several weeks, but a combination of heat and moisture may cause degradation to form soluble break-down products. Photographic gelatin layers are usually acidic in nature,15 Gelatin is an anisotropic substance: its properties are independent of geometry. If, for example, a stripped layer of gelatin is placed in water to observe lateral swelling, no preferential expansion is noted: a square piece remains square.
Our own work in examining the properties of gelatin was caused by observations made during experiments on the chemical restoration of discolored black-and-white negatives and prints. In the course of these experiments, which will be reported elsewhere, fifty year old experimental prints did not always survive' passing through a series of aqueous solutions at different pH values. In particular, when moving a print from a high acidic bath (pH 3.2) to an alkaline solution (pH 10.8), the gelatin layer occasionally separated from its support. Sometimes this happened during a subsequent washing stage.
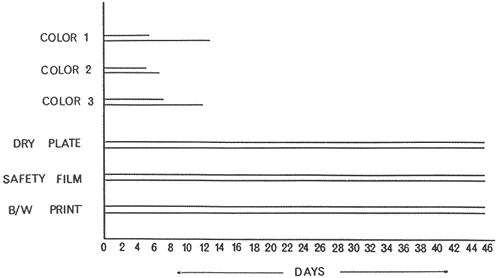
Consequently, some qualitative observations were collected by placing photographic samples (size 8 x 50mm, films and papers in black-and-white and colour, contemporary and historical) in deionized water (pH 6.5), or in Ottawa City tap water (pH~8). The time for. the first noticeable sign of deterioration was recorded, as was the time at which 50% or more of the sample had deteriorated. Further samples were pre-hardened using five different hardeners, and their effectiveness in extending the stability of the gelatin layers was determined. Results were recorded as shown in FIGURES 1 and 2.
FIGURE 1: Effect of soaking in water (pH~8) on the stability of gelatin layers. Soaking was done at about 26°C in the dark. The uppermost line ends when the first noticeable damage took place, and the lower line ends when the damage exceeded 50% of the surface areas. Black-and-white samples were intact after 46 days.
FIGURE 2: Effect of hardeners on resistance to soaking of a contemporary black-and-white film. Soaking was done at about 26°C in the dark. Lines are explained in Fig. 1. *Formula published by Saito.30
FIGURE 3: Melting point apparatus according to ANSI P114,11-1964. Samples are immersed in the melting point bath in small test tubes which are heated in a water bath. The 4 litre beaker serving as a water bath rests on a heating plate. The water bath is stirred mechanically. There is also a thermometer.
FIGURE 4: Spherical stylus scratch tester according to ANSI P111.37-1963.
The ability of hardeners to increase the resistance Of coated gelatin layers to soaking in water, which is seen in FIGURE 2, can be assessed more accurately by comparing the melting point of untreated gelatin with that of a hardened gelatin layer. This value--different from the definition of a melting point of a pure chemical compound--is the temperature at which the emulsion is destroyed, or leaves the support, in a rising- temperature melting point bath. Experimental details have been described by Crabtree and Hartt,16 who used the measurement of the melting point in determining the.- properties of various hardening fixing baths.17,18 Determining the melting point of a nonsupport layer of photographic materials became a specification of the American National Standards Institute (ANSI) in 1964.19 It has been reaffirmed several times since then. The experimental procedure described in the ANSI specification was used in this laboratory. The apparatus is shown in FIGURE 3. Determination of the melting point permits assessment of the effect of various hardeners on different types of gelatin coatings in historical photographic materials.
Another method of comparing the hardness of emulsions is based on scratch resistance measurements,20 in which a sapphire stylus under a 'given load is moved over the gelatin surface. The load is increased until visible scratches are produced. The scratch resistance is expressed in grams. Precursors of instruments used in this method were developed in the 1930s at the research laboratories of the Eastman Kodak Company.21 Scratch test methods and instruments equipped with a spherical stylus for abrading dry film have been described by Carroll and Paul in 1961.22 It subsequently became a standard method which was published as ANSI P111.37- 1963.23 FIGURE 4 shows the instrument used in our laboratory.
More important to the photograph manufacturer is the abrasion resistance of wet emulsions, and the test to determine it is called the mushiness test. Details of the test procedure and the emulsion mushiness apparatus were given by Parker and Sugeden.24It is also a standard method which was published as ANSI P114.35-1972.25 FIGURE 5 shows' the mushiness tester used in our work.
FIGURE 5: Test apparatus for mushiness according to ANSI P114.35-1972.
We found this test useful in determining the effect of chemical solutions, used in experimental restoration procedures, .on the stability of gelatin layers. FIGURE 6 shows the reproduction of two pairs of scratch lines produced on a 10 inch strip of photographic film under two different loads.
FIGURE 6: Two pairs of scratch lines produced on a contemporary film strip, length 10 inches.
Hardeners also affect the swelling of coated gelatin layers; generally causing deswelling in aqueous solutions. Green and Levenson26 described a practical swellmeter in 1972 which measures and records the swelling and deswelling of gelatin layers in photographic materials as they pass through processing solutions, or, relevant to our own work, through chemical solutions in experimental restoration cycles. While this property of gelatin is a complex one, which is affected by many factors, (such as the thickness of the dry gelatin layer, temperature, pH, salt concentration, etc.), our observations of the swelling behaviour of gelatin, using the instrument shown in FIGURE 7, have shown that Increased swelling in water is equivalent to a decrease in stability. In order to avoid breakup of the gelatin layer, its swelling beyond a certain thickness must be avoided, which can be achieved by varying any of the above mentioned parameters. Sheppard27 accurately described the gelatin's tendency to swell in water without limit as the temperature is raised, and to "take up all the water offered to it" until it virtually dissolves in it. FIGURE 8 shows the swelling behaviour with increasing temperature of a gelatin layer on a contemporary black-and-white film in demineralized water and in the ANSI melting point bath: in the highly alkaline salt solution the gelatin breaks down at about 90°c, while in water it continues to swell rapidly at the boiling point before the gelatin is destroyed.
FIGURE 7: The swellmeter according to Green and Levenson26 used in this work. The probe in the center is placed in a stainless steel bath which accommodates the various solutions in a treatment cycle. The amplifier is at left, the recorder at right.
FIGURE 8: Two swell curves of gelatin layers (swelling versus temperature) in a contemporary black-and-white film. See text for details.
The experiments described in this paper have demonstrated that the term "photographic gelatin" Is rather unspecific, and its properties, as mentioned earlier, are broad generalizations. Its reaction to various conditions, especially to hardeners, depends largely on the individual photographic record. No two manufacturers' products are alike, nor is the gelatin layer on a black-and-white film similar to that on a black-and- white paper of the same manufacturer. Historical materials behave differently than contemporary ones (layer thickness!), and black-and-white records differ from color materials. The various gelatin layers in a given color film or paper appear to have different characteristics (dependant upon the coupler and other additions embedded In it), and methods have been published for the selective removal of the individual emulsion layers -for analytical purposes.28 While Suydam and Skove reported a decrease in scratch resistance in microfilms processed with a hardener as compared to those processed without a hardener,29 we did not find such a clear relationship in historical materials. The effect of various hardeners on the abrasion resistance of gelatin layers was also discussed comprehensively by Saito.30
The described test methods are useful when comparing properties of gelatin layers on different types of materials. They can also serve to monitor the characteristics of a gelatin layer before and after a given treatment. The effect of specific hardeners on the properties of processed gelatin layers and their use preceding chemical restoration treatments has been determined. Observations were made regarding the relative stability of black-and-white films and black-and-white papers, and of black-and-white materials as compared to color materials. Exposure to some common fumigation agents (such as thymol, para-dichlorobenzene, ethylene oxide or methyl bromide) did not affect adversely the stability of coated gelatin layers.31 Water-soaked processed films and papers can safely be frozen and freeze-dried,32 with the important exception of glass plate negatives made by the wet collodion process. The properties of gelatin layers as determined by the described methods appear to be reversible within a fairly wide range of conditions. If driven beyond a threshold limit, abrupt deterioration is irreversible.
Mechanical drawings for the instruments shown in FIGURES 4 and 5 should be available, according to the corresponding ANSI standards, from the National Association of Photographic Manufacturers, Inc., in Harrison, N.J. However, detail drawings for these, two. apparatuses, as well as. for the swellmeter (see FIGURE 7( were obtained from the .Eastman Kodak Company in Rochester, N.Y. The three instruments were built by Otal Precision Co. Ltd of Ottawa, Ontario, Canada.
The authors wish to express their thanks to K.F. Foster, Director General of the Conservation and Technical Services Branch at the Public Archives of Canada, for his continued interest and support; to Brian Thurgood, of this laboratory, for valuable assistance in preparing the illustrations; to the Eastman, Kodak Company for supplying detail drawings for some test equipment; and to Diane Hopkins for help with the references and proofreading.
1. Ward, A.G. and Courts, A., editors. The Science and Technology of Gelatin. New York: Academic Press, 1977. 2 vol.
2. Sheppard, S.E. Gelatin in Photography. New York: Van Nostrand for Eastman Kodak Company, 1923. 2 vol.
3. Carroll, Burt H. "The Preparation of Photographic Emulsions" Journal of Chemical Education. 8(12):2341-2367; 1931 December.
4. Pouradier, J. "Photographic Gelatin" Journal fuer Signalaufzeichnungsmaterialien. 2(6):209-218; 1974.
5. Hill, Thomas T. "Photographic Gelatin and Synthetic Colloids for Emulsion Use" Journal of the Society of Motion Picture and Television Engineers. 77:1185-1183; 1968 November.
6. Hill, Thomas T. "Laboratory-Scale Photographic Emulsion Technique" Journal of Chemical Education. 43(9):492-493; 1966 September.
7. Hill, Thomas T. "The Literature of Gelatin" Literature of Chemical Technology. American Chemical Society, 1968. (Advances in Chemistry Series, Number 78) pp. 381-386. -
8. Sheppard, S.E. and Houck, R.C. "The Structure of Gelatin Sols and Gels I. The Viscosity of Gelatin Solutions" Journal of Physical Chemistry. 34: 273-298; 1930. (Kodak Research Laboratories, Communication, No. 395).
9. Sheppard, S.E. and McNally, J.G. "The Structure of Gelatin Sols and
Gels II. The Anisotropy of Gelatin Gels" Colloid Symposium Annual. 7:17-39; 1930. (Kodak Research Laboratories, Communication No. 399).
10. Sheppard, S.E. and Houck, R.C. "The Structure of Gelatin Sols and Gels III. Isoelectric Points of Gelatin" Journal of Physical Chemistry. 34:2187-2201; 1930. (Kodak Research Laboratories, Communication No. 433).
11. Adelstein, P.Z. and McCrea, J L. "Permanence of Processed Estar Polyester Base 'Photographic Films" Photographic Science and Engineering. 9(5):305-313; 1965 October.
12. Calhoun, J.M. and Leister, D.A. "Effect of Gelatin Layers on the Dimensional Stability of Photographic Film" Photographic Science and Engineering. 3(1) :8-17; 1959 January-February.
13. Current, Ira B. "Equipment for Testing Some Physical Characteristics of Sensitized Materials" Photographic Engineering. 5(4):227-233; 1954.
14. Schmitz, N. "Haltbarkeit Photographischer Schichten" (Permanence of Photographic Layers) International Kongress, Reprographie I. Helwich, O., ed. Cologne, 1963. pp. 74-76.
15. Schneider, W., Froehlich, A. and Schulze, H. "Die diffusionsechten Farbbildner des Agfacolorfilms" (The Non-Diffusing Dye couplers in Agfacolor Films) Die Chemie. 57(17/20);113-116; 1944 December 2.
16. Crabtree, J.I. and Hartt, H.A. "Some Properties of Fixing Baths" Transactions of the Society of Notion Picture Engineers. 13(38):364- 405; 1929. (Kodak Research Laboratories, Communication No. 396)
17. Crabtree, J.I. and Russell, H.D. "Some Properties of Chrome Alum Stop Baths and Fixing Baths" Journal of the Society of Motion Picture Engineers. 483-512, 667-700; 1930 May, June. (Kodak Research Laboratories, Communication No. 432)
18. Crabtree, J.I., Muehler, L.E. and Russell, H.D. "New Stop Bath and Fixing Bath Formulas and Methods for Their Revival" Journal of the Society of Motion Picture Engineers. 38:353-372; 1942.
19. American National Standards Institute. Determining the Melting Point of a Nonsupport Layer of Films, Plates, and Papers. New York: ANSI, 1964 December 2. (ANSI P114.11-1964) 7p.
20. Bernhardt, Ernest C. "A History of Hardness Tests Based on Scratch Resistance Measurements" ASTM Bulletin. 49-53;1949 March.
21. Sheppard, S.E. and Schmitt, J.J. "Measurement of Surface Hardness of Cellulose Derivatives" Industrial and Engineering Chemistry, Analytical Edition. 4(3):302-304; 1932.
22. Carroll, J.F. and Paul, JO "Test Methods for Rating Abrasion Resistance of Photographic Film" Photographic Science and Engineering. 5(5):288-296; 1961 September-October.
23. American National Standards Institute. Determining the Scratch Resistance of Processed Photographic Film. New York: ANSI, 1963, May 7. (ANSI P111.37-1963) 10 p.
24. Parker, J.T. and Sugden, L.J. "Determining the Resistance of Photographic Emulsions :to Damage During Processing" Photographic Science and Engineering 7(1) 41-47, 1963 February.
25. American National Standards Institute. Method for Determining the Resistance of Photographic Films to Abrasion During Processing. New York: ANSI, 1972 October 17. (ANSI P114.35-1972) 7p.
26. Green, A. and Levenson, G.I.P. "A Practical Swellmeter Journal of Photographic Science. 20:205-210; 1972.
27. Sheppard, S.E. "Behavior of Gelatin in the Processing of Motion Picture Film" Transactions of the Society of Motion Picture Engineers. 9(32): 707-727; 1927 (Kodak Research Laboratories, Communication No. 326).
28. Barchet, Hans-Martin "Eine Methode zur fermentiven Abloesung der einzelnen Emulsionsschichten von einem Mehrschichtenmaterial" (Method for the Fermentative Separation of the Individual Emulsion Layers of Multilayer Materials) Veroeffentlichungen des wissenschaftlichen Zentral-Laboratoriums der photographischen Abteilung- Agfa. 9:168-172, 1961.
29. Suydam, W.S. and Skove, A.G. "Scratch Resistance Parameters of Microfilm Emulsions" Proceedings of the National Micrographics Association. Tate, V.C. ed. 1964. pp. 49-59.
30. Saito, Takaya. "Firumu Zerachin Makumen no Kyôdo (Dai Ippô, Dai Nihô) 1. Hikkaki Shiken Hô ni yoru Sokutei 2. Kakushu Kômakuzai no Makumen Kyôkâ Kôka" (Strength of Gelatin Films (1st and 2nd Reports) 1. Measurement of Scratching Test 2. The Effect of Strengthening Gelatin Films of Various Hardeners) Tokyo Shashin Tanki Daigaku Kiyô. 37-59; 1964.
31. Hendriks, Klaus B. and Lesser, Brian. The Effect of Some Common Fumigation Agents on the Stability of Photographic Materials. Speech. Louisville, Kentucky: A.I.C. - Photographic Materials Group, 1984 February 3. (publication in preparation)
32. Hendriks, Klaus B. and Lesser, Brian. "Disaster Preparedness and Recovery: Photographic Materials" American Archivist. 46(1):52-68; 1983 Winter.