Journal of Food Science. Vol. 47.
1982. pp. 1241-44
Conditions for the
Formation of Heat-Induced Gels of Some Globular Food Proteins
Per Olof Hegg
Author Hegg is affiliated with the Dept. of Food Technology,
Univ. of Lund, Box. 740, S-220 07 Lund, Sweden.
Abstract
The quality of thermally induced aggregates of the globular
proteins conalbumin, serum albumin,  -lactoglobulin and lysozyme has been examined at various
salt concentrations and pH values. The properties of the aggregates
were characterized by their dry matter content. The results are
given as simple phase diagrams, The following areas of dry matter
content were found: solubility; transparent and opaque gels (dry
matter content of 5-9%); precipitates (dry matter content above 9).
Gels were formed only close to conditions of solubility. Only serum
albumin was found to be a protein with good gelling properties. A
small gelling area was registered for
-lactoglobulin and lysozyme has been examined at various
salt concentrations and pH values. The properties of the aggregates
were characterized by their dry matter content. The results are
given as simple phase diagrams, The following areas of dry matter
content were found: solubility; transparent and opaque gels (dry
matter content of 5-9%); precipitates (dry matter content above 9).
Gels were formed only close to conditions of solubility. Only serum
albumin was found to be a protein with good gelling properties. A
small gelling area was registered for  -lactoglobulin, while no gelling was observed for
conalbumin or lysozyme. under the conditions examined. No common
simple physical characteristic of the proteins used could be
correlated to good gelling behavior.
-lactoglobulin, while no gelling was observed for
conalbumin or lysozyme. under the conditions examined. No common
simple physical characteristic of the proteins used could be
correlated to good gelling behavior.
Introduction
the importance of gel formation in food is well known in, for
instance, many meat, egg and milk .product. In these products
proteins are considered to be the main texture building component.
The mechanism of protein gelling is, however, poorly understood and
theories on gel formation are instead to be found in carbohydrate
chemistry (c.f. Rees, 1972). It is conceivable, however, that some
of these theories could be applied also to protein chemistry.
It has been reported earlier that the egg white protein ovalbumin
forms excellent heat induced gels under certain conditions. These
conditions, however, must be exactly defined in terms of, for
instance, salt concentration and pH. In the case of ovalbumin
transparent gets are only found between pH 10 and 11 at
physiological salt concentration. At another salt concentration
transparent gels are formed within a different pH-interval (Hegg et
al., 1979). Other proteins seem either not to form gels by heating
(Hegg, 1978) or the conditions required for gel formation are
different when compared to ovalbumin.
This investigation was initiated to map the conditions required
for globular proteins to form thermally induced gels. It would be of
considerable value in food technology if a common environmental
condition or certain protein characteristic could be correlated with
the ability to form heat induced gels. To this end the following
common food proteins, which have different qualities in many
respects, were selected: conalbumin and lysozyme from egg white,
serum albumin from bovine blood, and  -lactoglobulin from milk. . It should be stressed that
the protein preparations used were extremely pure and well
characterized.
-lactoglobulin from milk. . It should be stressed that
the protein preparations used were extremely pure and well
characterized.
Experimental
Protein preparations
Bovine serum albumin (lot no. 16C-7201), conalbumin (lot no.
34C-8200) and lysozyme (lot no. 74C-8041) were all obtained from
Sigma Chemical Co. Serum albumin was free of bound fatty acids and
conalbumin from bound iron, as determined by differential scanning
calorimetry (Gumpen et al., 1979, Donovan and Rose, 1975). Both
preparations were saltfree. Lysozyme was desalted prior to use
according to Hegg et al. (1979).  -lactoglobulin was obtained saltfree by preparation from
flesh whole raw milk (Hegg, 1980). None of the used preparations
were contaminated by other proteins as determined by
SDS-polyacrylamide gel electrophoresis.
-lactoglobulin was obtained saltfree by preparation from
flesh whole raw milk (Hegg, 1980). None of the used preparations
were contaminated by other proteins as determined by
SDS-polyacrylamide gel electrophoresis.
Determination of water-holding
capacity
The experiments were performed at the fixed protein concentration
of 44 mg/ml. Protein concentrations were determined according to the
following:  -lactoglobulin A
1%/1cm=9.6 (Townend et al., 1960), serum albumin A 1%/1cm=6.67
Reynolds et al.. 1967), conalbumin A 1%/1cm=11.3 (Glazer and
McKenzie, 1963) and lysozyme A 1%/1cm,=26.9 (Ogasahara and
Hamaguchi, 1967). All values are given at the wavelength of 290
nm.
-lactoglobulin A
1%/1cm=9.6 (Townend et al., 1960), serum albumin A 1%/1cm=6.67
Reynolds et al.. 1967), conalbumin A 1%/1cm=11.3 (Glazer and
McKenzie, 1963) and lysozyme A 1%/1cm,=26.9 (Ogasahara and
Hamaguchi, 1967). All values are given at the wavelength of 290
nm.
Protein solutions were prepared as earlier described (Hegg et al.
1979). They were heated from 25-95° with a rate of 10°C/min.
Heat treatments were carried out in glass tubes (7 x 75 mm)
containing 1 ml of protein solution (Hegg et al. 1979). After
heating to 95 C the tubes were immediately cooled in ice water and
centrifuged at 30,000 x g for 45 min. The percentage of
thermally aggregated protein was calculated from the decrease in
absorbance of the supernatants at 280 nm. In most cases aggregation
was complete (> 90% insoluble protein) at 95°C
The water-holding capacity of the aggregates formed was
characterized by their dry matter (d.m.) content. The dry matter
content (%) was calculated from (C0 . V0
a)/(Wa) where C0 is the initial protein
concentration (mg/ml); V0. the initial solution volume
(ml); a, percentage aggregation; and Wa, the weight of
the aggregate (mg). A discussion of the d.m. content is given
elsewhere (Hegg et al., 1979), The reproducibility was ±3.5 %
calculated on the measured d.m. contents. In the few cases when
complete aggregation was not reached at 95°C the figure for d.m.
content is marked with an asterisk. Complete solubility was defined
as no observed phase separation after centrifugation.
Results
Conalbumin
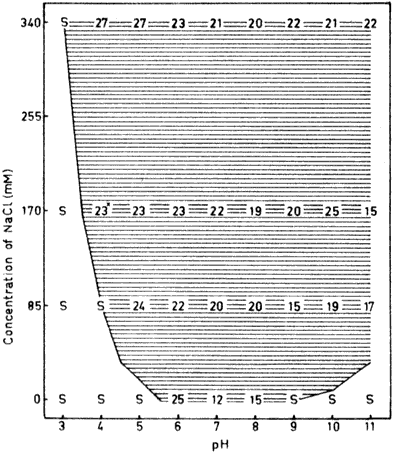
Fig. 1-The dry matter content (%) of conalbumin
aggregates formed through hearing to 95°C in different
concentrations of NaCl between, pH 3 and 11. Heating rate was
10°C/min. Dry matter contents are given with figures at each pH
for 0, 85 mM, 170 mM and 340 mM added NaCl. S indicates
solubility, The lines differentiate areas of different dry matter
content, /// 5-9% and=above 9%, The unhatched area indicates that
thermal aggregation does not occur. Dry matter contents when
aggregation was incomplete have been marked with an asterisk.
When no sodium chloride was added to the conalbumin solution,
thermally induced aggregates were only formed within the pH-interval
from around 6 to pH 85 (Fig. 1). Consequently,
aggregation in a saltfree protein solution only occurred around the
isoelectric point of the protein (approximately pH 6 for
conalbumin). The aggregates obtained were characterized by their dry
matter content and these values were all high in the pH-interval
described above. High dry matter values indicate that the aggregates
visibly appear as "white loose precipitates" which are effectively
packed together by centrifugation. No transparent gels, which are
characterized by dry matter values of around 5% or opaque gels with
dry matter contents between 5 and 9% were observed.
The limits between solubility and aggregation gradually moved
towards lower and higher pH-values. respectively, when the salt
concentration was increased. As can be seen from the incomplete
aggregation near the boundary (dry matter figures marked with an
asterisk) there was a gradual transition from solubility to complete
aggregation. With the exception of a few points. the dry matter
values in Fig, 1 are all above 13, i.e. the
aggregates visibly appeared as precipitates. No systematic tendency
to a decrease or increase in dry matter was found with changing
conditions. The quality of heat induced aggregates of conalbumin
thus seem to be largely independent of salt concentration and
pH.
The very steep course of the solubility/aggregation line observed
for conalbumin at acidic pH-values was not observed earlier for
ovalbumin. The boundary between solubility and aggregation seems to
be closely related to the titration curve of the protein (Hegg,
1919). Conalbumin has a pH-induced transition between pH 3.5 and 5,
and thus a profound alteration in the amount of titratable groups in
this pH interval (Wisnia et al., 1961).
Serumalbumin
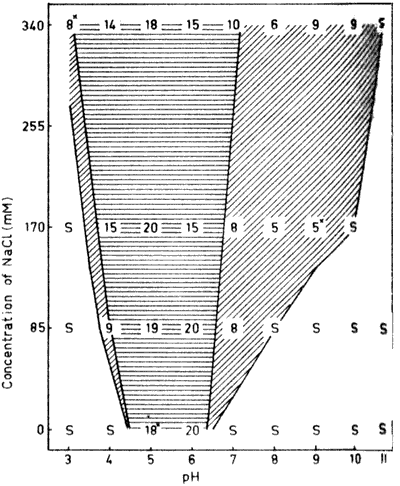
Fig. 2 . The dry matter content (%) of serum
albumin aggregates formed through heating to 95°C in n different
concentrations of NaCl between pH 3 and 11, For details, see legend
to Fig. 1.
Heat induced aggregation in saltfree solution of serumalbumin
occurred within a more narrow pH-interval than for conalbumin. The
isoelectric point is located around the middle of this interval (Fig 2).
Serum albumin has a similar pH-induced transition at acidic pH
values as conalbumin. Thus, the course of the boundary between
solubility and aggregation at acidic pH values is the same for these
two proteins. At alkaline pH-values they differ, however, and serum
albumin is soluble under conditions ranging front pH 6.5 in saltfree
solution to 11 in NaCl. This difference compared to conalbumin is
mainly due to the fact that serum albumin undergoes structural
transitions also at alkaline pH-values (Steinhardt and Reynolds,
1969).
The most important difference between the two proteins is,
however, the extremely large gelling area for serum albumin at
neutral and weak alkaline pH-values (Fig. 2). At
physiological salt concentration for instance, gels are formed in
the wide pH-interval 6.-9.5. Both transparent and opaque gels are,
found within this interval. Transparent gels are characterized by a
dry matter content around 5% and these gels are nearly always found
close to the boundary between solubility and aggregation. This is s
probably due to the higher net charge repulsion between the protein
molecules required for the formation of a gel with transparency
(Hegg, 1978).
Precipitates of serum albumin were always observed near the
isoelectric point regardless of the salt concentration used. This is
in agreement with the results on conalbumin Progressively lower dry
matter contents were obtained towards the solubility/aggregation
limit on the acidic side and even at these acidic pH-values a narrow
gelling area could be distinguished. A gradual decrease in dry
matter content was also registered from the isoelectric point
towards alkaline pH-values. This tendency was, however, casually
broken at pH 8, where lower values were found at all salt
concentrations. The reason for this is probably due to the
structural transition mentioned above.
 -Lactoglobulin
-Lactoglobulin
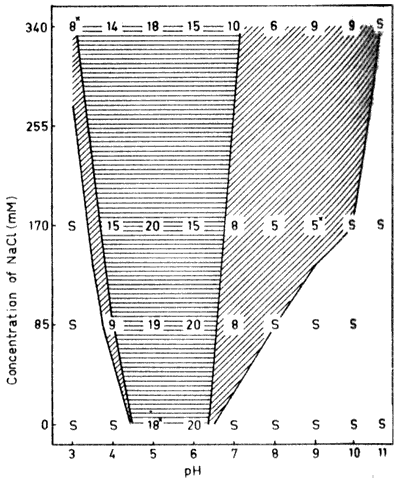
Fig. 3. The dry, matter content (%) of  -lactoglobulin aggregates formed
through heating to 95°C at different concentrations of NaCl
between pH 3 and 11. For details, see legend to Fig.
1.
-lactoglobulin aggregates formed
through heating to 95°C at different concentrations of NaCl
between pH 3 and 11. For details, see legend to Fig.
1.
 -Lactoglobulin is the
most abundant whey protein and has an isoelectric point of around
5.2 (McKenzie, 1971). The protein has a pH-induced transition above
pH 7 (McKenzie et al., 1967), which accounts for the solubility
characteristics (Fig. 3) similar to those
registered for serum albumin The solubility/aggregation boundary at
acidic pH-values more reflects the shape of a normal titration
behavior (Hegg, 1978).
-Lactoglobulin is the
most abundant whey protein and has an isoelectric point of around
5.2 (McKenzie, 1971). The protein has a pH-induced transition above
pH 7 (McKenzie et al., 1967), which accounts for the solubility
characteristics (Fig. 3) similar to those
registered for serum albumin The solubility/aggregation boundary at
acidic pH-values more reflects the shape of a normal titration
behavior (Hegg, 1978).
Gel formation occurred close to the aggregation/solubility
boundary both in on the acidic and alkaline side of the isoelectric
point. Both these areas of gel formation were however, very narrow.
Furthermore, only opaque gels were obtained. The behavior earlier
found for ovalbumin (Hegg, et al., 1979) that at any salt
concentration the dry matter contents were at highest around the
isoelectric point and then gradually decreased towards lower and
higher pH values was observed also for  -lactoglobulin. Compared to the other proteins in this
investigation, the dry matter contents were generally lower for
-lactoglobulin. Compared to the other proteins in this
investigation, the dry matter contents were generally lower for  -lactoglobulin and dry matter
values above 20% were found only in saltfree solotion.
-lactoglobulin and dry matter
values above 20% were found only in saltfree solotion.
Lysozyme
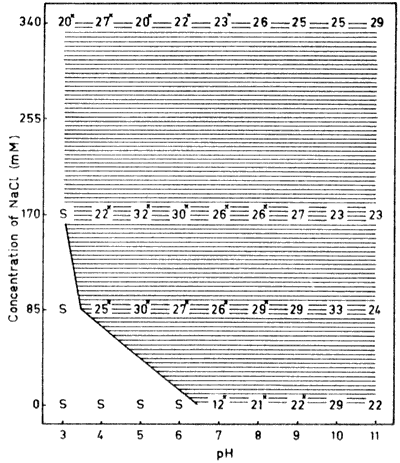
Fig. 4. The dry matter content (%) of lysozyme
aggregates formed through heating to 95°C In different
concentrations of NaCl between pH 3 and 11. For details, see legend
to Fig. 1.
Lysozyme is the smallest globular protein used in this
investigation with a molecular weight of 14,500 (Dayhoff, 1972).
The protein has a small solubility area, and is soluble only on the
acidic side of the isoelectric point (Fig. 4).
This is due to the high isoelectric point (pH 10.5-11.0) of the
protein (Gilbert, 1971).
The dry matter contents found in the, aggregates were all very
high, i.e. only precipitates were formed. No gel formation, either
of transparent or opaque gels, could be detected. Furthermore,
there was an extremely slow transformation from solubility to
complete precipitation for lysozyme. Several of the dry matter
values in Fig. 4 are marked with an asterisk
which indicates incomplete aggregation,
Discussion
The solubility areas for the proteins given in Fig. 1-4 show the
same general appearance. From the isoelectric point, these areas
expand with a decreasing or increasing pH and shrink with an
increasing salt concentration. Thus, thermal non-aggregating
conditions are largely determined by the state of ionization of the
titratable groups of the proteins, and the differences found in
extension of the boundaries between solubility and aggregation
mainly reflect different net charges of the proteins under various
conditions (Hegg, et al., 1979). The, effect of salt and pH on the
,, attractive forces is small in comparison with the effect on the
repulsive net charge. Consequently, it would be possible to predict
the boundaries between solubility and aggregation for any globular
protein by knowing titration curves, amount of salt present in the
sample and simple physical data, as the, isoelectric point,
pH-induced transitions etc.
Ovalbumin has earlier been reported to be a protein with good
gelling properties, i.e. gels were formed at a wide pH- and salt
concentration range (Hegg et al., 1979). Serum albumin (Fig. 2) has gelling properties comparable to those
of ovalbumin. The gelling area for serum albumen is, however,
displaced towards lower pH values and higher salt concentrations and
the area where transparent gels were formed was also more
narrow.
 -Lactoglobulin has an
isoelectric point close to that of serum albumin and a similar
pH-induced transition at alkaline pH values. For
-Lactoglobulin has an
isoelectric point close to that of serum albumin and a similar
pH-induced transition at alkaline pH values. For  -lactoglobulin, however, only a
very small gelling area was detected (Fig. 3),
Obviously these simple physical characteristics could not be used as
a correlation to good getting property of a protein.
-lactoglobulin, however, only a
very small gelling area was detected (Fig. 3),
Obviously these simple physical characteristics could not be used as
a correlation to good getting property of a protein.
Areas of gel formation could not be distinguished for conalbumin
(Fig. 1) or lysozyme (Fig.
4). Our data do not indicate a simple physical characteristic in
common to account for their lack of ability to form gels thermally.
It must be pointed out however, that only a fixed protein
concentration of 4.4% has been used in this investigation. A higher
concentration would possibly facilitate the formation of a
three-dimensional protein network and thus the formation of a gel
structure. It cannot be excluded that a critical concentration for
gelling exists for every protein and that this concentration in the
case of conalbumin and lysozyme is very high. The kinetics of
heating is another factor that affect the gelling behaviour A low
rate of heating generally has a positive effect on gel formation
(Hegg, 1978). From these points of view the experimental conditions
selected in this investigation are quite unfavorable for gel
formation since they were designed to sort out a protein with good
gelling properties from a poor one.
Disulphide bridges and sulphydryl groups have been suggested to
be important for crosslinking of Proteins. The proteins used in this
investigation differ widely in their content of these groups and
thus no correlation between disulphide or sulphydryl content and the
ability to form thermally induced gels was found. Some other
proteins apart from those reported on here have also been
investigated. Notably, myoglobin containing no disulphide bridge or
free sulphydryl group (Mailer and Cordes, 1966), was found to be an
extremely potent gelling protein, further indicating that these
parameters are unimportant in gel formation of these proteins. The
forces which keep the framework of the gel together must instead be
found in the hydrophobic and hydrogen bonds, which become available
during thermal denaturation of the globular proteins. These forces
counteract the repulsive net charge of the proteins, and a delicate
balance between attractive and repulsive forces seems to be a
prerequisite for the formation of a gel framework (Hegg et al.,
1979). The differences in the ability to form gels might reflect
different types of intermolecular interactions in the aggregates of
the proteins examined. It has been proposed that ß-sheet
hydrogen bonding might be important in aggregate formation (Clark et
al., 1981). The ß-sheet content in the native state of serum
albumin, for instance, is reported as low and that of ß
-lactoglobulin as high (Wasylewski, 1979). There is therefore no
indication that a high content of intramolecular ß -sheet
structure in the native state would facilitate the formation of
intermolecular ß-sheet formation in the denatured state.
Instead the potent gelling proteins serum albumin, ovalbumin and
myoglobin seem to have a high helical content in the native state
(Joly, 1965; Chen et al., 1974). Since the poor gelling protein
lysozyme also has a high helix content (Chen et al., 1974) this
seems not to be decisive factor in gel formation either.
The contribution from the hydrophobic forces to the formation of
the gel framework is difficult to assess since hydrophobicity is not
easy either to calculate or to measure. None of the proteins used
are known to have an extreme content of hydrophobic amino acids.
Furthermore, the location of the hydrophobic amino acids within the
molecule is probably more important for the development of
intermolecular interactions than the total content. Further
speculation in this matter is not meaningful, since except for
lysozyme, the protein structures for the model proteins examined are
unknown.
In conclusion, it seems possible to predict the conditions
required for aggregation and solubility of a globular protein but
hard to identify a special protein characteristic which is crucial
for gel formation. If gel formation occurs, however, this is found
close to the boundary between aggregation and solubility.
Studies at various protein concentrations might possibly add
further clues to the mechanism of protein gelling. As no
characteristic property of the proteins used in this investigation
could be correlated with a good gelling behaviour it could not be
excluded that most proteins might form thermally induced gels. If
so, the question of gelling might be reduced to find the right
condition for the actual protein
References
Chen, Y.H., Yang, J.T., and Chau. K.H. 1974.
Determination of the helix and ß-form of proteins in aqueous
solution by circular dichroism. Biochemistry 13: 3350.
Clark, A.H., Saunderson, D.H.P., and Suggett, A.
1981. infrared and laser-Raman spectroscopic studies of
thermally-induced globular protein gels. Int. J. Peptide Protein
Res. 17: 353.
Dayhoff, M.D. (Ed.) 1972. "Atlas of Protein
Sequence and Structure." Vol.. 5. The National Biomedical Research
Foundation Washington, DC.
Donovan. J.W. and Rose, K.D. 1975. Iron binding
to conalbumin Calorimetric evidence for two distinct species with
one bound iron atom. J. Biol. Chem. 260: 6026.
Gilbert, A.B. 1971. Egg albumen and its
formation. Physiol. Biochem. Dom. Fowl 3: 1291.
Glazer, A.N, and McKenzie, H.A. 1963. The
denaturation of proteins. 4. Conalbumin and iron (III)-conalbumin in
urea solution. Biochim. Biophys, Acta. 71: 109.
Gumpen, S., Hegg, P.O., and Martens, H. 1979.
Thermal stability at fatty acid-serum albumin complexes studied by
differential' scanning calorimetry. Biochim. Biophys. Acta 574:
189.
Hegg P.O. 1978. Thermal aggregation and
denaturation of egg white proteins. A model study of food protein
behaviour, Ph.D. thesis, Univ. of Lund, Lund, Sweden.
Hegg, P.O. 1980. Thermal stability of  -lactoglobulin as a function of
pH and the relative concentration of sodium dodecylsulphate. Acta
Agric. Scand. 30: 401.
-lactoglobulin as a function of
pH and the relative concentration of sodium dodecylsulphate. Acta
Agric. Scand. 30: 401.
Hegg, P.O., Martens, H, and Löfquist,, B.
1979. Effects of pH and neutral salts on the formation and quality
of thermal aggregates of ovalbumin. A study on thermal aggregation
and denaturation. J. Sci. Food Agric. 30: 981.
Joly, M. 1966. A physico-chemical approach to the
denaturation of proteins", p. 279. Academic Press, New York.
Mahler, H.R. and Cordes, E.H. 1966. In
"Biological Chemistry," p. 104. Harper and Row, New York.
McKenzie, H.A, 1971, In "Milk Proteins. Chemistry
and Molecular Biology," Vol. 2, Academic Press, New York.
McKenzie, H.A., Sawyer, W.H., and Smith, M.B.
1967. Optical rotatory dispersion and sedimentation in the study of
association-dissociation: bovine  -lactoglobulin near pH 5. Biochim. Biophys. Acta. 147:
73.
-lactoglobulin near pH 5. Biochim. Biophys. Acta. 147:
73.
Ogasahara. K. and Hamaguchi, K. 1967. Structure
of lysozyme. 1. Effect of pH on the stability of lysozyme. J.
Biochem. 61: 199.
Rees, D.A. 1972. Polysaccharide gels. A molecular
view. Chemistry & Industry 19: 630.
Reynolds, J.A., Herbert, S., Polar, H., and
Steinhardt, J. 1994 The binding of divers detergent anions to bovine
serum albumin. Biochemistry 6: 937.
Steinhardt, J. and Reynolds, J.A. 1969. In
"Multiple Equilibria in Proteins." Academic Press, New York.
Townsend, R., Weinberger, L., and Timasheff, S.N.
1960. Molecular interactions in  -lactoglobulin. 4. The dissociation of
-lactoglobulin. 4. The dissociation of  -lactoglobulin below pH 3.5. J.
Am. Chem. Soc. 82: 3175.
-lactoglobulin below pH 3.5. J.
Am. Chem. Soc. 82: 3175.
Wasylewski, Z. 1979. Protein-cationic detergent
interaction. Fourier transform infrared and laser raman
spectroscopic studies on the interaction between proteins and
dodecyl pyridinium bromide. Act. Biochim. Polonica 26: 205.
Wishnia, A., Weber, 1., and Warner, R.C. 1961.
The hydro[illegible] equilibria of conalbumin. J. Am. Chem. Soc. 83:
2071.
Ms received 11/27/81; revised 2/24/82; accepted 3/3/82.
I thank Gunnel Lundh and Sigrid Häggström for technical
assistance. This investigation was supported by grant No. 78-3756
from the. Swedish Board of Technical Development.
 -lactoglobulin and lysozyme has been examined at various
salt concentrations and pH values. The properties of the aggregates
were characterized by their dry matter content. The results are
given as simple phase diagrams, The following areas of dry matter
content were found: solubility; transparent and opaque gels (dry
matter content of 5-9%); precipitates (dry matter content above 9).
Gels were formed only close to conditions of solubility. Only serum
albumin was found to be a protein with good gelling properties. A
small gelling area was registered for
-lactoglobulin and lysozyme has been examined at various
salt concentrations and pH values. The properties of the aggregates
were characterized by their dry matter content. The results are
given as simple phase diagrams, The following areas of dry matter
content were found: solubility; transparent and opaque gels (dry
matter content of 5-9%); precipitates (dry matter content above 9).
Gels were formed only close to conditions of solubility. Only serum
albumin was found to be a protein with good gelling properties. A
small gelling area was registered for  -lactoglobulin, while no gelling was observed for
conalbumin or lysozyme. under the conditions examined. No common
simple physical characteristic of the proteins used could be
correlated to good gelling behavior.
-lactoglobulin, while no gelling was observed for
conalbumin or lysozyme. under the conditions examined. No common
simple physical characteristic of the proteins used could be
correlated to good gelling behavior.


