Journal of Food Science. Volume 37. (1972). p.965-66
A Research Note
The amount water adsorbed by a mixture of components, as in a food system, to a first approximation is the sum of the water bound by the component parts, principally the salts and protein (Berlin et al., 1968). Therefore the amount of water bound by proteins is of some concern to those interested in equilibrium moisture content of protein foods. This note reports on the quantitative measurement of water binding of some food proteins, with emphasis on the oil-seed proteins. By "water binding" is meant the water vapor adsorbed by a dried protein powder, after equilibration against water vapor of known relative humidity, which in this case was 84%.
[The?] casein, bovin serum albumin and dried egg were purchased from Sigma. Soybean isolates were obtained from Central Soya ("Promine D") and Grain Processing Corp. ("Profam 90 H/S"). Cottonseed isolates were prepared by the two-step method of Berardi et al. (1969) from glandless cottonseed. Coconut protein was prepared by precipitation at pH 5.0 after extraction of fresh coconuts at pH 8 (Hagenmaier, et al., 1972). Peanut protein was precipitated at pH 4.5 after extraction of full-fat peanuts at pH 8.0 (Rhee et al, 1972). Fish protein concentrate was prepared from deboned [illegible]ake by hot isopropyl alcohol extraction (Aberdeen Pilot Plant). Sunflower protein was prepared by the method of Gheyasuddin et al. ) by extraction at pH 10.5 and precipitation at pH 5.0, without the alcohol wash.
The peanut and coconut samples were initially rather high in oil content (8% and 25%), and were consequently hexane extracted to achieve oil contents of less than 1%. All samples subjected to extended dialysis to remove hydrophilic salts. The pH values of the liquid ions were adjusted to the desired value and the entire suspension freeze dried in each case. Total nitrogen and amide nitrogen con[tents?] of all freeze-dried samples were measured he results are included in Table 1.
Water binding was measured as weight up-after exposure of the dry protein sample [illegible] water vapor of 84% relative humidity (over [saturated?] KCl), after the method of Mellon et al., 1947). Our measurements showed that equilibrium was achieved within 24 hr. Measurements of weight uptake were consequently after 24-48 hr of equilibration. the reported results are the averages of measurements made on at least three different samples of each protein. All protein samples remained in the solid state after equilibration: they did not form glasses or solutions.
Protein solubility was measured in separate experiments. To measure solubility the freeze-dried protein sample was added to water to make a 1% suspension, the pH maintained at the desired value for 45 min (with intermittent stirring), then the suspensions centrifuged, filtered, and the dissolved protein determined from Kjeldahl analysis of the filtrate. Each reported solubility is the average of at least two measurements.
Amide nitrogen was determined by the method of Chibnall et al. (1958).
The amino acid contents used in calculations were from the literature, with the exception of peanut and coconut, which were measured in this laboratory. The sunflower analysis was from Gheyasuddin et al. (1970); the cottonseed analyses were from Martinez et al. (1970); the soybean was averaged from several sources: company literature, Circle and John-son (1958) and FAO (1970). the eggwhite, casein and fish analyses were from FAO (1970), and the serum albumin results were from Spahr and Edsall (1964).
| Protein sample | Bound water (g water ------ 16g N)a |
% N of dry sampleb |
% of N which is amide NC |
% of protein dissolved at pH 6.0d |
|---|---|---|---|---|
| Cottonseed isolate I | 22.4 | 15.7 | 11.9 | 84 |
| Coconut | 21.6 | 15.8 | 6.5 | 37 |
| Soybean | ||||
| "Profam 90/HS" | 20.3 | 15.5 | 8.7 | 40 |
| "Promine D" | 19.2 | 16.2 | 9.7 | 22 |
| Peanut | 17.4 | 17.1 | 10.1 | 48 |
| Sunflower | 17.3 | 16.5 | 11.4 | 8 |
| Cottonseed isolate II | 16.0 | 18.0 | 9.2 | 5 |
| Serum albumin | 29.5 | 15.7 | 4.1 | 100 |
| Egg white | 24.0 | 15.4 | 7.0 | 94 |
| Casein | 21.6 | 15.5 | 8.6 | 90 |
| Fish (FPC) | 21.0 | 16.4 | 5.5 | 6 |
a. Standard deviation is 0.7.
b. Standard deviation is 0.1.
c. Standard deviation is 0.2.
d. Standard deviation is 2.5.
a. At 84% relative humidity, standard deviation is
0.7.
b. In 1% aqueous suspension, standard deviation is
3%.
THE DATA in Table 1 give the measured values for amide nitrogen and total nitrogen. Also shown are the values for water adsorbed by protein samples adjusted to pH 6.0 before freeze drying, and amount of protein dissolved at pH 6.0. The results are arranged in Table 1 so that the first seven entries are oilseed proteins and the last four are animal proteins.
The data in Table 2 reflect the influence of pH on water binding and solubility. for the water binding results, the pH values pertain to the aqueous suspensions that were freeze dried to give the dry protein samples. for the solubility experiments, the pH values pertain to aqueous suspensions in which solubility was measured. All pH values are accurate to ± 0.2 pH units.
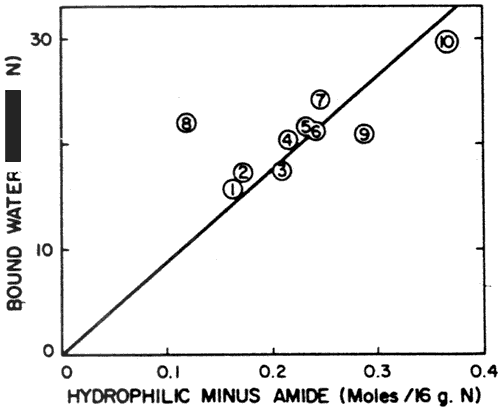
Fig. 1--Dependence of water binding of proteins on moles of hydrophilic groups minus moles of amide groups for (1) Cottonseed II; (2) Sunflower; (3) Peanut; (4) Soybean (Pro Fam 90/HS); (5) Casein; (6) Coconut; (7) Egg white; (8) Cottonseed I; (9) Fish and (10) Serum albumin.
THE RESULTS in Table 1 indicate that the animal proteins are generally lower in amide nitrogen and bind more water than the oilseed proteins. The animal proteins are also generally more soluble--with the [illegible]tion of the fish sample, which is [considered?] to be a denatured protein. The [ranking?] of the oilseed proteins is in order of decreasing water binding. This ranking is for 84% relative humidity, but should hold at all high values of relative humidity. Literature values for water binding of proteins (Bull, 1944) at different water activities indicate, that for relative humidities not greatly different, proteins may be ordered according to water binding capacity at one relative humidity, and that the same order holds at other relative humidities.
The data in Table 2 indicate that pH (of an aqueous suspension of the protein sample) has little effect on water adsorption. The protein solubility, on the other hand, shows the expectedly large dependence on pH. This contrasting dependence on pH indicates that there is not a good correlation between water binding and protein solubility.
The amino acid contents and amide nitrogen values were used to calculate the amount of hydrophilic groups of the different proteins. The hydrophilic groups were taken as hydroxyl plus carboxyl plus basic groups. It should be pointed out that protein carboxyl groups are not merely the sum of glutamic acid and aspartic acid values as normally reported in amino acid analyses, because these values normally include their amides, glutamine and asparagine. The amide nitrogen must be subtracted out to give the true aspartic and glutamic acid contents. For the data in Figure 1 the number of amide groups is subtracted out a second time so that "hydrophilic minus amide groups" is doubly dependent on the amount of amide nitrogen.
The data in Figure 1 indicate that the trend is for increased water binding with larger values of hydrophilic minus amide groups. This relationship was first noted by Bull and Breese (1968). This dependence on amide nitrogen is especially significant for the oilseed proteins, because of their generally large amount of amide nitrogen. The data suggest that deamidation of the oilseed proteins might substantially increase their water binding potential, and consequently render these proteins more valuable for food applications that demand a more hygroscopic protein.
Berardi, L.C., Martinez, W.H. and Fernandez, C.J. 1969. Cottonseed protein isolates: Two step extraction procedure. Food Technol. 23(10): 75.
Berlin, E., Anderson, B.A. and 1968. Comparison of water vapor sorption by milk powder components. J. Dairy Sci. 51: 12.
Bull, H.B. 1944. Adsorption of water vapor by proteins. J. Amer. Chem. Soc. 66: 1499
Bull, H.B. and Breese, V. 1968. Protein hydration: 1. Binding sites. Arch. Biochem. Biophys. 128: 488.
Chibnall, A.C., Mangan, J.L. and Reese, 1958. Studies on the amide and C-terminal residues in proteins. Biochem. J. 68: 111.
Circle, S.J. and Johnson, D.W. 1958. Edible isolated soybean protein. In "Processed Plant Protein Foodstuffs," ed. Altschul, A.M. Academic Press, New York.
FAO. 1970. Amino acid content of food and biological data on proteins.
Gheyasuddin, S., Cater, C.M. and Mattil, K.P. 1970. Preparation of a colorless sunflower protein isolate. Food Technol. 24(3): 242.
Hagenmaier, R.D., Cater, C.M. and Mattil, K.F. 1972. Critical unit operations in the aqueous processing of fresh coconuts. J. Amer. Oil Chem. Soc. 49: 178.
Martinez, W.H., Berardi, L.C. and Goldblatt, L.A. 1970. Cottonseed protein products--Composition and functionality. J. Agr. Food Chem. 18: 961.
Mellon, E.F., Korn, A.H. and Hoover, S.R. 1947. Water adsorption of proteins. 1. The effect of free amino groups in casein. J. Amer. Oil Chem. Soc. 69: 827.
Rhee, K.C., Cater, C.M. and Mattil, K.F. 1972. Simultaneous recovery of protein and oil from raw peanuts in an aqueous system. J. Food Sci. 37: 90.
Spahr, P.F. and Edsall, J.T. 1964. Amino acid composition of human and bovine serum mercaptalbumins. J. Biol. Chem. 239: 850.
Ms received 5/28/72; revised 7/15/72; accepted 7/16/7 2.