Present address Canada Dept. Of Agriculture Experiment Station Summerland. B.C., Canada
THE PHYSICAL and chemical changes involved to alterations of meat quality, particularly tenderness, have been studied extensively during the past 70 years (Bate-Smith, 1948; Hamm, 1960; Dyer and Dingle, 1961, Fennema and Powrie, 1964, Szczesniak and Torgeson 1965). Although it is believed that collagen in meat from beef can contribute a certain amount to a "background toughness" (Barley, 1972), to fish meat collagen does not seem to participate greatly in the toughening process since fish muscle contains less connective tissue, only 3-4% vs. 2-27% of the total protein for mammals (Hamoir, 1961), and the collagenous material In the white muscle dissolves upon cooking and remains as a soft white mass after cooling.
Decreases in the amounts of proteins extractable with 7% lithium or potassium chloride solutions from beef and rabbit muscle which had been stored at temperatures from 0-35°C and fish muscle kept at a storage temperature of -3°C were first reported by Smith (1933), Bate-Smith (1937), Reay (1933). Reay and Kuchel (1936). However, undesirable changes to quality of cod. particularly transitions from tenderness to toughness were first correlated with the decreasing extractability of the actomyosin fraction by Dyer (1951) Luijpen (1957) found the solubility tests did not distinguish as well as organoleptic tests between cod samples frozen at -20°C and -30°C, but the correlation was very good for samples stored at -10°C In beef the solubility of the fibrillar proteins were related to shearpress and taste panel evaluations (Hegerty et al., 1963) Weinberg and Rose (1960) found that the post rigor increase in extractability of the contractile proteins of chicken meat paralleled an increase in tenderness while Khan et al (1963) showed that the solubility of chicken muscle proteins decreased more rapidly during storage at -10°C than at -18°C. Using extraction methods somewhat different from those of previous workers, Goll et al. (1964) did not find any relation between protein solubility and tenderness
Another approach toward the mechanisms of textural alterations was followed by Locker (1960), who related sarcomere length of the myofibrils to tenderness. These findings were confirmed by Herring et al (1965) who found that as the postmortem muscles shortened there were corresponding decreases to sarcomere length, increases in fiber diameter and decreases to tenderness, and if the excised muscles were restrained from postmortem contraction they would remain more tender. In view of the sliding filament model (Huxley, 1963; Huxley and Brown, 1967) in which the two major structural proteins, actin and myosin, are arranged in a highly organized superlattice of thin and thick filaments, their postmortem interaction at diminishing ATP concentrations, the increasing cell disorganization with the breakdown of membrane integrity, the failure of the calcium pump in the absence of ATP and the unrestricted movement of ions including those of heavy metals are some of the factors which gradually lead to the random interaction between cell constituents. These mechanical considerations then also make it tempting to relate the extent to which the thick filaments, equipped with "cross bridges" overlap or interdigitate with the thin filaments during the shortening phase of the contraction process and during rigor mortis (Huxley, 1956) to the chemical aspects of protein-protein interactions and to meat texture. Despite the fact that the extractability tests were not found entirely suitable for practical applications, the study of particularly the two major structural proteins actin and myosin, their self-associating behavior as well as the product of their interaction, the actomyosin complex, would appear necessary for an understanding of meat texture and the possibility of its chemical manipulation
Ultracentrifugal studies of the mechanisms involved in the "spontaneous aggregation" of myosin in solution were first carried out by Holtzer (1956). This aggregation phenomenon had been a recognized problem accompanying myosin purifications from their earliest beginnings (Portzehl et al. 1950). Further physical studies on the aggregation of myosins, including that from fish, were carried out by Holtzer and Lowry (1959), Connell (1959, 1960) and Johnson and Row, (1961) who generally studied the rate of the decreasing monomeric myosin concentration with increasing time. Results from these experiments showed that myosin aggregation at 0°C increased with increasing protein concentration, was at a minimum around pH 7-8, and increased with increasing ionic strength. The sulfhydryl (SH) groups of myosin were also investigated as possible contributors to the aggregation reaction; however it was concluded that they were not involved in the bonding which holds the myosin aggregates together The reasons for thus conclusion were given as follows: (1) The numbers of SH groups measured with N-ethylmaleimide did not change during 7 days of storage at 0°C and myosin reacted with N-ethylmaleimide (blocked SH groups) aggregated as rapidly as untreated myosin, (2) Monothioglycol at 0.01 M did not inhibit aggregation (Connell, 1959) and at a concentration of 0.1M monothioglycol rather accelerated aggregation (Connell, 1960).
Investigations to elucidate the nature of the interactions between proteins which may contribute to the structural properties of meats and other foods were therefore continued.
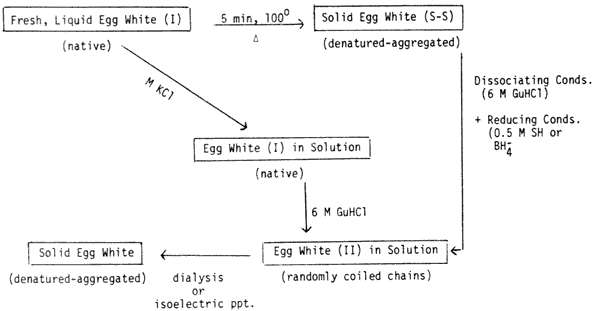
>Fig 1-Schematic diagram of the various physical
states of egg white. Heat transforms liquid egg white, which
consists of 54% ovalbumin, an SH protein into a solid, firm or
gelatinous form This denatured, aggregated egg white ( -structure, fully
extended peptide chain) cancer be solubilized in a nonhydrolytic way
at room temperature by salt (KCl) solutions which are acidified (ph
1.0) or made basic (pH 12.0) nor by 6M guanidine hydrochloride
(GuHCl) solution, (pH 10.0).
-structure, fully
extended peptide chain) cancer be solubilized in a nonhydrolytic way
at room temperature by salt (KCl) solutions which are acidified (ph
1.0) or made basic (pH 12.0) nor by 6M guanidine hydrochloride
(GuHCl) solution, (pH 10.0).
In order to bring the solidified or gel like structure of the egg white (S-S) back into solution, conditions are required which will dissociate hydrophobic, hydrogen and electrostatic bonds as well as bring about reduction of covalent SS bonds which have formed during the heating process. These dissociating and reducing conditions are provided by a solution which is 6M in GuHCl 0.5M in mercaptoethanol and at pH 9.0-9.5. When sodium borohydride is used as a reducing agent, the pH is maintained at 10.5 with M NaOH. In the dissociating reducing solvent the egg white proteins are present as randomly coiled polypeptide chains and can be regenerated back to a solid, denatured form by dialysis or by dilution and acidification (isoelectric precipitation). Dialysis of "Egg White (II)" against a solution of KCl containing reducing agent will permit the proteins to regain the native form of "Egg White (I)"
Solid age white from a "5 min egg" (4g) was chopped into small particles about 0.5 on edge and was then suspended in 30 ml of 6M guanidine HCl which had been adjusted with 1.0N KOH to pH 10.5 The particles only swelled in this solution and the opaque white changed to a somewhat more translucent appearance during 24 hr of storage at room temperature After the addition of reducing agent, 3 x 0.2g, portions of NaBH4 and readjusting the pH with KOH, the protein began to dissolve to a clear solution within about 1 hr. Addition of an octanol methyl-n-hexylcarbonol was used to reduce foaming problems.
Using mercaptoethanol as the reducing agent instead of NaBH4, the solution was prepared so that it was 6M in guanidine HCl, 0.5M in mercaptoethanol and was then adjusted to between pH 8 5 and 9.0. After dissolution, the protein could be precipitated again by dialysis or by dilation and acidification
The 6M guanidine HCl solution was pre pared by dissolving 17.28g of guanidine HCl ('ultra pure' reagent from Schwarz-Mann) in about 10 ml of water, adding 2.2 ml N KOH and making final adjustments to pH 10.5 with 5 and 1N KOH before the volume was made up to 30 ml with water. If the solution was to be 6M in guanidine MG as well as 0.5M in mercaptoethanol. 17.28g of guanidine HCl were dissolved in about 10 ml of water and 2.2 ml N KOH, 1.17g mercaptoethanol and two pellets (about 0.3g) of sodium hydroxide were added and dissolved before adjusting the volume to 30M with water. The pH of this latter solution was then generally around pH 9.0. Sodium bisulfite was not a suitable substitute for NaBH4 or mercaptoethanol due to its relatively low solubility in the already concentrated solution. However, sodium bisulfite has been used previously in combination with sodium dodecylsulfate at 100°C in the dispersion of 60-80% of chicken feather keratin (Lundgren and O'Connell, 1944).
Lingcod muscle was cooked m a microwave oven or pressure cooker. About 5g of this cooked meat was then chopped into small pieces, extracted two times with diethyl ether and evacuated with a water pump to remove residual ether. The fibrous material was then suspended in 30 ml of 6M guanidine HCl adjusted [illegible] 10.5. After about 30 min of standing [illegible] temperature a gel had formed which [could?] be broken gradually by the addition of mercaptoethanol adjusted to pH 9.0 but more effectively with NaBH4 at pH 10.5. The resulting solution, however, remained turbid and had to be clarified by centrifugation at about 20,000 x G. After centrifugation a layer of material, presumably lipoprotein, was floating at the top of the tube This material constituted 4% of the weight of the original meat The protein dissolved in the guanidine-reducing solution could be brought out of the solution back into the solid state by dialysis or acidification to the isoelectric point of the proteins.
Kamaboko gel (about 4g) appeared to dissolve to some extent in 30 ml 6M guanidine HCl solution at pH 10.5. However, after 24 hr the dissolution was still incomplete. To completely dissolve the material the 6M guanidine HCl solution had to be also 0.5M in mercaptoethanol at about pH 9.0. Lipoprotein material causing turbidity in the solution floated to the top within 24 hr or could be removed by centrifugation. On dialysis the protein precipitated again and remained as a white foam-mat type of material after freeze drying.
Beef (cooked as roast) was cut into 0.5 cm cubes. When 2.5g were added to 30 ml of the guanidine, HCL-mercaptoethanol solution at pH 9.0, the meat particles became translucent and turned a blood-red color as the dissociating reducing solution penetrated the meat, dissolved the proteins and at the same time reduced the ferric ion of myoglobin to the ferrous state in myoglobin. After 10 hr, about 36% of the initial material was still undissolved and consisted mostly of red muscle fibers embedded in collagenous material. When this material was finely ground up, more complete dissolution was achieved with only small amounts (1-2%) of presumably collagen and elastin, as judged from their blue-white efflorescence (Ex 360 nm) remaining. It was therefore concluded that the degree of dispersion must be related to the rate of solution particularly of the preparations containing hydrophobic the membranous lipid materials and collagenous or cross linked proteins,
Myosin was prepared, aggregated and redissolved as previously described (Buttkus, 1970, 1971).
Fig 2-Relative rates of decreasing protein concentration myosin solutions stored at 0, -10 and -30°C for up to 14 days (solid lines). The myosin solutions were 0.72% in protein, 0.45M in KCl, buffered with 0.026M potassium phosphate at pH 6.9 After freezing, the protein solutions were thawed in 4-5°C water centrifuged at 12,000 X G and the remaining protein in the supernate was measured The differences between the dashed and solid lines indicate the ranges of denaturation contributed by the catalysis of heavy metal contamination, (about 2 X 10-4%) present in 'reagent grade' KCl, above the aggregation caused by the concentration effect of the protein during freezing. The values for the dashed lines were derived from Fig. 3 and superimposed on the rates of myosin aggregation at different temperatures and consequently different salt concentrations. For instance, in the liquid regions of the myosin solution frozen to -10°C, the KCl concentration can be expected to be about 3.2 molar (from a temperature-composition phase diagram of KCl) In the solution held at 0°C no concentration of the salt or protein, will take place.
THE EXPERIMENTS of Connell (I959, 1960) tested for the involvement of the SH groups in the denaturation mechanism of myosin by adding mercaptans as protective agents after the extraction of the protein. However, the negative results did not exclude the function of the SH groups from a more complex series of mechanisms in which other forms of molecular interactions as well are participating in the aggregation phenomena. For instance, a molecular alteration brought about by the formation of disulfide (SS) bonds followed by a rearrangement of hydrophobic and hydrogen bonded regions on an intra- and intermolecular basis during denaturation and aggregation could not be reversed by SS bond reducing agents alone, but would have to include conditions which would also break intermolecular hydrophobic and hydrogen bonds. From our studies on aggregated myosins, it was concluded that such complex interactions are indeed involved and must be very similar to those in heat denatured egg while (Fig. 1). However, selective conditions for the reversal of the "random" noncovalent bonding in the aggregated state or from the randomly coiled peptide chains to the organized arrangement in the native protein have not been worked out for egg white or myosin as if has in the case of lysozyme (Tanford et al., 1966).
In contrast to the denatured-aggregated myosin which requires hydrophobic, hydrogen as well as SS bond breaking reagents for its unfolding, in the depolymerization of the enzyme urease only SS bond reducing reagents seem to be required as the polymeric species can be reversed to a monomeric form by the addition of low concentrations (0.01M) of reducing agent (Creeth and Nichol, 1960). In gelatin primarily hydrogen bonds between the polypeptide chains are responsible for its water including gel structure which can be ruptured already at 20°C upon the addition of 2M salt (KCl) solution pH 5-7 or by heating to higher temperatures in the absence of solvent. The structure of acid precipitated casein is believed to he held primarily by electrostatic bonding and can be dissociated again in 1M KCl solution at pH 12 to a milky solution. Extensive studies on gelatin interactions and the casein micelle formation are recorded in the literature (Harrington and Rao, 1970, Waugh, 1961). Yet another type of aggregation takes place in the case of tobacco mosaic virus protein, whereby monomers aggregate with a release of water molecules when the temperature is raised from 5°C to 25°C and the reaction is reversed on lowering it (Lauffer et al., 1958).
While previous workers had concentrated their studies on the monomeric native myosin remaining n solution after denaturation, in more recent work (Buttkus, 1970), the properties of the insolubilization, aggregated or polymeric myosin which can readily be centrifuged out of solution were studied. In a high ionic strength solution of 0.5M KCl the insolubilization of myosin from rabbit or trout muscle proceeded at a faster rate when the solution was frozen than at 0°C (Fig. 2). To reverse the reactions of aggregation and bring the insoluble aggregated proteins back into solution, two general types of conditions had to be provided namely, one to break or weaken the non covalent associations. particularly the hydrophobic and hydrogen bonds, using guanidine HC1, and the second to break the covalent SS bonds by the use of reducing agents such as mercaptoethanol sodium borohydride. While native myosin will unfold and dissociate into randomly coiled peptide chains in 8M urea solution the aggregated polymeric myosin, which is insoluble in salt solutions, redissolved in 6M guanidine hydrochloride (pH 8.0-10.5) only after the addition of reducing agents. These results indicated the involvement of SS as well as noncovalent hydrogen, hydrophobic and possibly ionic bonds inn the formation of the polymeric myosin. From the changes in the SH content of a myosin solution during storage, the initiating mechanism in the myosin polymerization was postulated to involve first an intramolecular SS bond formation by oxidation of up to 5-6 of the 42 SH groups in the native, monomeric molecules (5 x 105g per mole) and proceeded then by intermolecular SH-SS exchange reactions involving the remaining free sulfhydryl groups on neighboring molecules. It can be visualized that during the denaturation processes the hydrophobic and hydrogen bonds buried in the interior of the protein molecule become exposed and broken from their native arrangement following conformational changes in coiled or helical sections of the peptide chains (Morawetz, 1972), and reform in a manner different from those in the native structures, possibly also intermolecularly.
Fig. 3-Effect of different salt concentrations 0.5-3.3M 'reagent grade' KCl, on the aggregation of rabbit myosin during storage periods of 3 days (open circles) and 12 days (triangles) at 0°C The initial protein concentration in the different samples, was 0.75% (7 5 mg protein per ml.). Myosin prepared with salt solution which had previously been passed over a cheating resin (Chelex 100) showed very little to no aggregation 0 5-3.2M KCl after 14 days of storage at 0°C (squares).
The results of previous workers that N-ethylmaleimide or low concentrations of reducing agents had no protective effects on myosin aggregation was confirmed in our studies. However, these tests cannot be considered conclusive evidence for the critical involvement of SH groups in the denaturation mechanism since it is known that without the addition of denaturing agents, such as urea or guanidine, only a portion of the SH groups of myosin are available for reaction with these agents (Buttkus, 1971), Myosin molecules on the other hand [may?] exert an action like denaturing [agents?] upon their neighbors and gain thereby access into the interior of each other. Further, the fact that relatively low concentrations of mercaptoethanol produced an acceleration of myosin aggregation strengthened our belief that after a few days of storage or sometimes already during or after preparation some myosin molecules contained SS bonds. These considerations were in agreement with the experiments of Huggins et al (1951), who, using plasma albumin or ã-globulin, both proteins containing SS as well as SH groups, showed that the addition of small amounts of thiols enhanced the rate of intermolecular SH-SS exchange, leading to the formation of polymers connected by SS bonds.
Studies on the rate of aggregation of myosin in frozen solution showed that it proceeded fastest at -10°C in the vicinity of the eutectic point of the potassium chloride solution which was used to solubilize the native protein On lowering the temperature from the freezing point down to the eutectic point, the freezing of water as ice results in the concentration of solutes such as buffer, salts as well as proteins, and reaches an optimum effect at the eutectic point. Since the rate [of the] molecular or multimolecular reactions [are] concentration dependent, the finding that the maximum rate of aggregation of myosin was around -10°C, the eutectic point of the solvent solution, was in agreement with the theories of Kiovsky and Pincock (1966) who had shown that for systems for which the kinetics were already known at ambient temperatures the accelerated rates in frozen solutions could be predicted on the basis of the concentration of solute in the liquid regions of the apparently solidly frozen system. The proposed mechanism for the accelerated rate of aggregation of the salt-soluble contractile muscle proteins in the liquid regions of the frozen solution is further supported by the findings of Connell (1960) who showed that the rate of myosin aggregation at 0°C increased with increasing protein concentrations
The results of our investigation on the effect of salt (KCl) on myosin aggregation agreed essentially with those of earlier publications (Holtzer and Lowey, 1959; Connell, 1960) and showed that increasing concentrations of reagent grade KCl increased the rate of myosin aggregation (Fig. 3). However, myosin prepared with the greatest possible care to exclude trace metal contamination, from reagents (i.e., iron content of crystalline KCl was 1 x 10-6%), glassware and water with an electrical resistance of 18 megohms-cm did not show this effect of increased rate of aggregation with increasing salt concentration. It was further shown (Buttkus, 1971) that some of the trace metals present in reagent grade chemicals formed mercaptides with the SH groups of myosin and, while some of the metals could be removed with chelating agents, the total number of SH groups was generally 5-6 moles SH per 5 x 105g of protein lower than in myosins prepared with reagents which had previously been purified by passage over chelating resins. The decrease in SH groups detectable with the Ellman reagent in the presence of chelating agent was therefore attributable to a metal catalyzed formation of SS bonds- These SS bonds are then able to undergo exchange reactions with the remaining SH groups in neighboring molecule. to form intermolecular covalent bonds, a major step in the aggregation polymerization process
An important consideration to this SH-SS exchange mechanism during the denaturation of myosin is that once 2-6 SH groups of the original 42 per molecule have been oxidized, the polymerization process can proceed without any further decrease in measurable SH groups because for every SH group which then becomes incorporated into an SS bond another SH or mercaptide group (S-) is generated by an established mechanism (Cecil end McPhee. 1959).
RSSR + R'S- 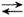 RSSR' + RS-
RSSR' + RS-
RSSR' + R'S- 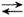 R'SSR' + RS-
R'SSR' + RS-
A representative example of the rate of myosin denaturation or insolubilization at different temperatures is shown in Figure 2. The protein was dissolved in KCl solution prepared with 'reagent grade' KCl (containing 1 x 10-4% iron) and glass-distilled water. The contribution of metal ions to aggregation in the eutectic point region (-10°C) was obtained from Figure 3 by taking the difference between the amount of protein aggregate formed after 12 and 14 days of storage at 0°C in solutions of 3.2M 'reagent grade' KCl and 3.2M 'Chelex 100' treated KCl which contained 1 x 10-6 iron and was dissolved in 'Millipore' Super -Q water of lowest conductivity (18 megohms-cm). As can be seen in Figure 2, aggregation of the protein myosin due to metal contamination from the reagents plus the denaturation due to the concentration effect of the protein in frozen solution is represented by the solid line while the denaturation in solutions with a 100x reduced metal contamination, broken lines, represents the level of myosin aggregation caused primarily by the concentration effect on the protein during freezing. The difference is about 8% of the total protein (7.2-8.0 mg/ml) originally present in solution and does not alter the relative order of the aggregation rates during freezing, -10° > -20° > -30° > 0°C.
The mechanisms and types of bonds outlined above in the
denaturation and aggregation of purified myosins appeared to be of a
more general nature and were shown to be also involved in the
denaturation and aggregation processes during the cooking of foods
containing sulfhydryl proteins such as egg white, kamaboko or whole
muscle. The cooked products, which were firmer and had a greater
shear strength than the raw materials (Buttkus, 1963) could be
solubilized in 6M guanidine HCl only in the presence of reducing
agents such as mercaptoethanol or borohydride, indicating the
critical involvement of covalent disulfide as well as nonresident
hydrophobic, hydrogen and probably tonic bonds in the firming
process of these foods during heating. In order to achieve the
dissolution of cooked, firm egg white in about 1 hr at 20°C, the
dissociating solution was made 0.5M in the reducing agent in
mercaptoethanol. The sodium borohydride reagent was used at a
concentration of about 2$. (0.4-0.5M). Sodium sulfite was not a
preferred reducing agent due to its poor solubility characteristics
in the already concentrated GuHCl solution. Figure
1 shows a schematic diagram of the various physical states in
which egg white, composed of the various proteins [ovalbumin 54%,
conalbumin 13%, ovomucoid 11%, lysozyme 3% and others Feeney, 1964)]
can exist and the necessity of reducing agents to cleave the SS
bonds of the solidified, hard or denatured egg white as well as
reagents to provide conditions for the dissociation of hydrophobic
and hydrogen bonds to bring about dissolution. The heat denatured,
solidified egg white would not dissolve in 2M KCl containing
reducing agents, neither will it dissolve at room temperature in
salt solutions which have been acidified to pH 1 or made basic to pH
12. The solubility properties of heat denatured egg white therefore
appear similar to those of denatured myosin and a substantial
fraction of the keratin, has also been reported to yield to
extraction at 40-50°C with saturated area containing reducing
agents. By x-ray analysis. regenerated keratin, once of interest to
the textile industries, and heat denatured egg white were both found
to have a  -keratin type, unfolded or denatured structure (Wormell
and Happey, 1949). The formation of SS bonds during the heating of
dilute solutions of ovalbumin had also been suggested by Halwer
(1954) and our evidence that reducing conditions are required in the
dissolution of the solid egg white further supports the earlier
findings of Hamm and Hofmann (1965) that SS bonds are formed during
the cooking of meat and that they possibly contribute to the firming
or toughening in the cooking process. The unique elastic and
cohesive properties if wheat proteins have also been attributed to
their disulfide bonds (Nielsen et al., 1962). On the other hand. a
gel containing 12% gelatin, a sulfur-free protein having a low
content of amino acids with hydrophobic side chains, readily
dissolved at room temperature in 2M KCl or 8M urea, and no reducing
agent was required.
-keratin type, unfolded or denatured structure (Wormell
and Happey, 1949). The formation of SS bonds during the heating of
dilute solutions of ovalbumin had also been suggested by Halwer
(1954) and our evidence that reducing conditions are required in the
dissolution of the solid egg white further supports the earlier
findings of Hamm and Hofmann (1965) that SS bonds are formed during
the cooking of meat and that they possibly contribute to the firming
or toughening in the cooking process. The unique elastic and
cohesive properties if wheat proteins have also been attributed to
their disulfide bonds (Nielsen et al., 1962). On the other hand. a
gel containing 12% gelatin, a sulfur-free protein having a low
content of amino acids with hydrophobic side chains, readily
dissolved at room temperature in 2M KCl or 8M urea, and no reducing
agent was required.
Whole tissues containing membranous and other lipid material were much more difficult to dissolve than egg white, kamaboko, or denatured myosin, probably due to poor penetration of the aqueous solvent into the lipid material containing also insoluble residues such as collagen and elastin. Particularly in fishery products some insolubility of lipid-containing material after long or poor storage conditions may also be due to the interaction of the proteins with autoxidation products from polyunsaturated lipids such as aldehydes, ketones, peroxides and epoxides, resulting in products which are cross-linked and have solubility properties similar to the naturally cross linked Proteins collagen or elastin (Buttkus, 1970). Similar effects on the solubility of proteins are also produced by formaldehyde, a product from the reduction of trimethylamine oxide in some species of fish (Castell et al., 1973)
When, therefore, raw or cooked fish muscle slices were dissolved under the above dissociating and reducing conditions of 6M guanidine HCl and 0.5M mercaptoethanol or NaBH4 some differences were noted in comparison with the behavior of cooked egg white and denatured myosin. The solutions obtained after reduction and dissociation of the SS and noncovalent bonds were turbid instead of clear as the ones obtained from egg white. The lipid material could, however, be separated from the remaining solution upon centrifugation when a lipid or lipid-protein layer floated to the top. The Protein in solution could then be reclaimed as a precipitate on acidification or dialysis. Due to poor penetration of the solvent into the tissue, the speed of dissolution of cooked muscle, particularly that from beef, was very much dependent on its degree of dispersion, i.e. the finer emascerated the muscle, the faster it would dissolve. Cooked egg white would go into solution readily when it was chopped up to about 0 5 cm cubes and left in the dissociating reducing solution 1 hr at 20°C
That 95% of undenatured protein can be extracted from fresh fish muscle with 0.85M NaCL after homogenization in a Waring Blender has been reported by Dyer et al. (1950). Tomlinson and Geiger (1963) have shown that, if the extraction is carried out in metallic (stainless steel) vessels, quite large decreases in the extractable protein occurred. A reduction in the rate of myosin aggregation in the presence of polyphosphates (Buttkus, 1970) and the successful use of polyphosphates in the 'tenderization' of meat (Ellinger, 1972) suggest a general mechanism involving chelation of metal ions by the phosphates. The addition of polyphosphates to protein solutions can therefore prevent further formation of SS bonds but it cannot cleave those which have already formed. In terms of whole meat systems this would mean that addition of polyphosphates can prevent further toughening involving the form than of SS bonds, but could not make extensively cross linked or tough meat tender.
Observations made on the SH groups of the purified proteins myosin and actomyosin during their preparation storage and denaturation (Morales and Hotta, 1960, Blum, 1960; Buttkus, 1971) appeared to be in general agreement with the behavior of the natural products containing sulfur proteins such as egg white and meat from fish and mammals. Metal catalyzed oxidation of the SH groups and a reversal by cleavage of some of the SS bonds also by metals (Cecil and McPhee 1959) appears to be responsible for the erratic results which are sometimes obtained in the measurement of the SH content. This has been particularly demonstrated in actomyosin preparations where fluctuations within a relatively short time period cycled from 6.0 to 5,0 and back to 6.0 SH groups per 105g of rabbit myosin B (Blum, 1960). For chicken actomyosin variations from 9.8 to 8.7 and back again to 9.4 SH per 105g with a standard deviation as high as ±0.8 have been reported by Hay et al. (1972). At an approximate molecular weight of 5 x 105g of protein for myosin, a decrease by about 1.0-1.2 SH groups per 105g would indicate that about 5-6 SH groups have been oxidized to 2.5-3.0 SS bonds per molecule and the presence of SS bonds in the protein molecules would then enable them to react by intermolecular SH-SS exchange reactions with the remaining SH groups of other molecules to form polymeric material. In work on myosin, prepared with purified reagents (Chelex 100 treatment), a standard deviation of ±0.11 moles SH per 105g was generally attained with the Ellman procedure which made it possible to measure the SH content per myosin molecule with a variation of not more than ±0.55 SH residues. these values are in agreement with work on actomyosin, prepared with purified reagents, where SH per 105g of protein were measured with s standard deviation of ±0.1 (Jacobson and Henderson, 1971).
Bailey, A J. 1972. The bans of meat texture, J. Sci. Fd. Agric. 23:995.
Bate-Smith, E.C. 1937. Native and denatured muscle proteins. Proc,. Roy. Soc London. B124:136.
Bate-Smith, E.C. 1948. The physiology and chemistry of rigor mortis, with special reference to the aging of beef. Adv. Food Res. 1:1.
Blum. J.J. 1960 Interaction between myosin and its substrates. Arch, Biochem. Biophys. 87:104.
Buttkus, H 1963. .Apparatus tat measuring the energy input in cutting fibers of fish muscle. J. Fish. Res. Bd. Canada 20:181.
Buttkus, H. 1979 Accelerated denaturation of my[illegible] frozen solution. J. Food. Sci. 25:55:[illegible]..
Buttkus, H. 1971. The sulfhydryl content of rabbit and trout in relation to protein stability Can. J. Biochemistry 49:97.
Castell, C H., Smith, B and Dyer. W J., 1973. Effects of formaldehyde on salt extractable proteins of Gadoid muscle. J. Fish Res. Bd. Canada. 30:1205.
Cecil, R and McPhee, J.R. 1959 The sulfur chemistry of proteins. Adv. Protein Chem. 14:255.
Connell, J.J. 1959 Aggregation of cod myosin during frozen storage. Nature 183:664
Connell, J.J. 1960 Studies on the proteins of fish skeletal muscle. Denaturation and aggregation of cod myosin. Biochem. J. 75:530.
Creeth. J.M. and Nichol, L.W. 1960. Evidence for the chemical interaction of urease in solution. Biochem J. 77:230
Dyer, W.J.. French. H.V. and Snow. J.M. 1950. Proteins in fish muscle 1. Extraction of protein fractions in fresh fish. J. Fish. Res. Ed. Canada 7:585
Dyer, W.J. 1951 Protein denaturation in frozen stored fish. Food Res. 16:522.
Dyer, W.J. and Fraser, D.I. 1959. Protons in fish muscle J. Fish Res. Bd. Canada 16:43.
Dyer, W J. and Dingle. J.R. 1961. Fish proteins with special reference to freezing. In "Fish as Ford." Vol. 1, Ed. Borgstrom, G., p.275, Academic Press, New York.
Ellinger, R.H 1972. "Phosphates as Food Ingredients." The Chemical Rubber Co., CRC Press. Cleveland. Ohio.
Feeney. R.E- 1964, Egg proteins. In "The Proteins and their Reactions" Ed. Schultz,. H.W. and Anglemier, A F.. p.209. The Avi Publishing Co. Inc.
Fennema, O. and Powrie, W.D. 1964. Fundamentals of low temperature food Preservation. Adv. Food Res. 13:219
Goll. D.E., Henderson. D.W. and Kline. E.A. 1964 Postmortem changes in physical and [chemical] properties of bovine muscle. J. F[illegible]29:590.
Halwer. [illegible], 1954. Disulfide crosslinks in denatured ovalbumin. J. Am. Chem. Soc. 76: 183.
Hamm, R. and Hofmann, K. 1965. Changes in the sulfhydryl and disulfide groups in beef muscle protein during heating. Nature 207:1269.
Hamm, R. 1960. Biochemistry of meat hydration. Adv. in Food. Res. 10:355.
Hamoir, G. 1961 Distribution of protein nitrogen in rabbit and fish muscle. in "Biochemist's Handbook," Ed. Long, C , p. 673. E, and F.N Spon Ltd., London W.C.2
Harrington, W.F. and Rao, N.V. 1970. Collagen structure in solution. 1. Kinetics of helix regeneration in single chain gelatins. Biochemistry 9:3723.
Hay, J.D. Currie, R.W., and Wolfe, F.H. 1971. The effect of aging on physiochemical properties of actomyosin from chicken breast and leg muscle. J. Food Sci. 37:346.
Hegarty, G R.. Bratzler, L J add Pearson. A.M. 1963 The relationship of some intracellular protein characteristics to beef muscle tenderness. J. Food Sci. 28:525.
Herring. H K, Cassens, R.G, end Briskey, E.J. 1965. Studies on bovine muscle tenderness as influenced by carcass position, sarcomere length and fiber diameter. J. Food. Sci. 30:1049
Holtzer, A 1956 On the spontaneous aggregation of myosin. Arch. Biochem. Biophys. 64:507.
Holtzer. A. and Lowey. A. 1959. The aggregation of myosin. J, Am. Chem. Soc. 81:1378
Huggins, C., Tapley. D.F. and Jansen, E V. 1951. Sulfhydryl-disulfide relationships in the induction of gels in proteins by urea. Nature 167 592.
Huxley. H.E. 1956. Muscular contraction. Endeavour 15:177,
Huxley, H.E. 1963- Electron microscope studies on the structure of natural of synthetic protein filaments from striated muscle. J. Mol Biol. 7:281.
Huxley. H.E. and Brown, W, 1967. Low angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J. Mol. Biol. 30:383.
Jacobson, A.L. and Henderson, J. 1971. Auto-oxidation of actomyosin Can. J. Biochem. 49:1264
Johnson. P. and Rowe, A.J. 1961. The spontaneous transformation reactions of myosin. Biochem. Biophys. Acta 53: 43.
Khan, A.W. van den Berg, L. and Lent, C.P. 1963. Effects of frozen storage on chicken muscle proteins, J. Food So., 28. 425
Kiovxky, T.E. and Pincock, R.E. 1966. Mutarotation of glucose m frozen aqueous solutions. J. Am. Chem. Soc. 88:4704.
Lauffer, M A., Ansevin,. A.T., Cartwright. T.E. .and Brinton, C.C. 1958. Polymerization-depolymerization of tobacco mosaic virus protein. Nature 181:1338.
Locker., R.H. 1960. Degree of muscular contractions as a factor in tenderness of beef. Food Res. 25:304
Luijpen, A.F.M.G. 1957, Denaturation of fish proteins. Nature 180:1422.
Lundgren, H.P. and O'Connell, R.A. 1944. Artificial fibers from Corpuscular and fibrous proteins. Ind. Eng. Chem. 36:770.
Morales, H. and Hotta, K. 1960 The adenosine
triphosphatase activity of myosin B treated with S- -aminoethylisothiouronium. J. Biol. Chem. 235:1979.
-aminoethylisothiouronium. J. Biol. Chem. 235:1979.
Morawetz, H. 1972. Conformational transitions in macromolecules. Adv. Protein. Chem. 26:243
Nielson, H.C. Babcock, G.E. end Senti, F.R. 1962. Molecular weight studies on glutenin before and after disulfide bond splitting Arch. Biochem. Biophys. 96.252
Portzehl, H. Schramm, G. Weber, H.H. 1950. Aktomyosin und seine Komponenten. Z. Naturfosch. 5b:61
Reay, G.A. 1933. The influence of freezing temperatures on haddock's muscle. J. Soc. Chem. Ind. 52:265
Reay. G.A. and Kuchel C.C. 1936. The proteins of fish. Food Invest, Bd. Rept., p.93.
Smith, E.C 1933. Physiology of muscle protein. Ann. Rept. Food Invest. Bd. p.19
Szczesniak, A.S. and Torgeson, K.W. 1965. Methods of meat texture measurement viewed from the background factors affecting tenderness. Adv. Food Res. 14:33,
Tanford, C. Pain. R.H. and Otchin. N.S. 1966. Equilibrium and kinetics of the unfolding of lysozyme by guanidine hydrochloride, J. Mol. Biol. 15:489.
Tomlinson. N. and Geiger, SE. 1963. The bound nucleotides of freshly frozen and severely denatured lingcod muscle. J. Fish Bd. Canada 21:187.
Waugh, D.F. 1961 Casein interactions and micelle formation. J. Phys. Chem. 65:1793.
Weinberg. B. and Rose, D. 1960. Changes in protein extractability during post-rigor tenderization of chicken breast muscle, Food Technol. 14:376.
Wormell. R.L. and Happey, F. 1949. Regenerated keratin fiber, Nature 163:18.
Ms received 9/4/73; revised 10/24/73; accepted 10/30/73.